This document presents an overview of several common methods for analyzing proteins:
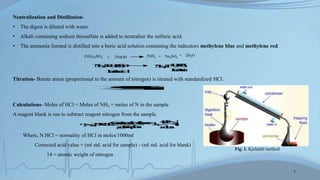
The Kjeldahl method involves digesting proteins with sulfuric acid to convert nitrogen to ammonium sulfate, then distilling and titrating to determine protein content. The Biuret method uses a color reaction between copper ions and peptide bonds to quantify proteins. The Lowry method combines the Biuret reaction with reduction of Folin-Ciocalteu reagent by amino acids for high sensitivity. Other methods discussed include measuring UV absorption at 280nm and color reactions with ninhydrin or in turbidimetric assays. The document compares the principles, procedures, applications, and advantages/disadvantages of each approach.