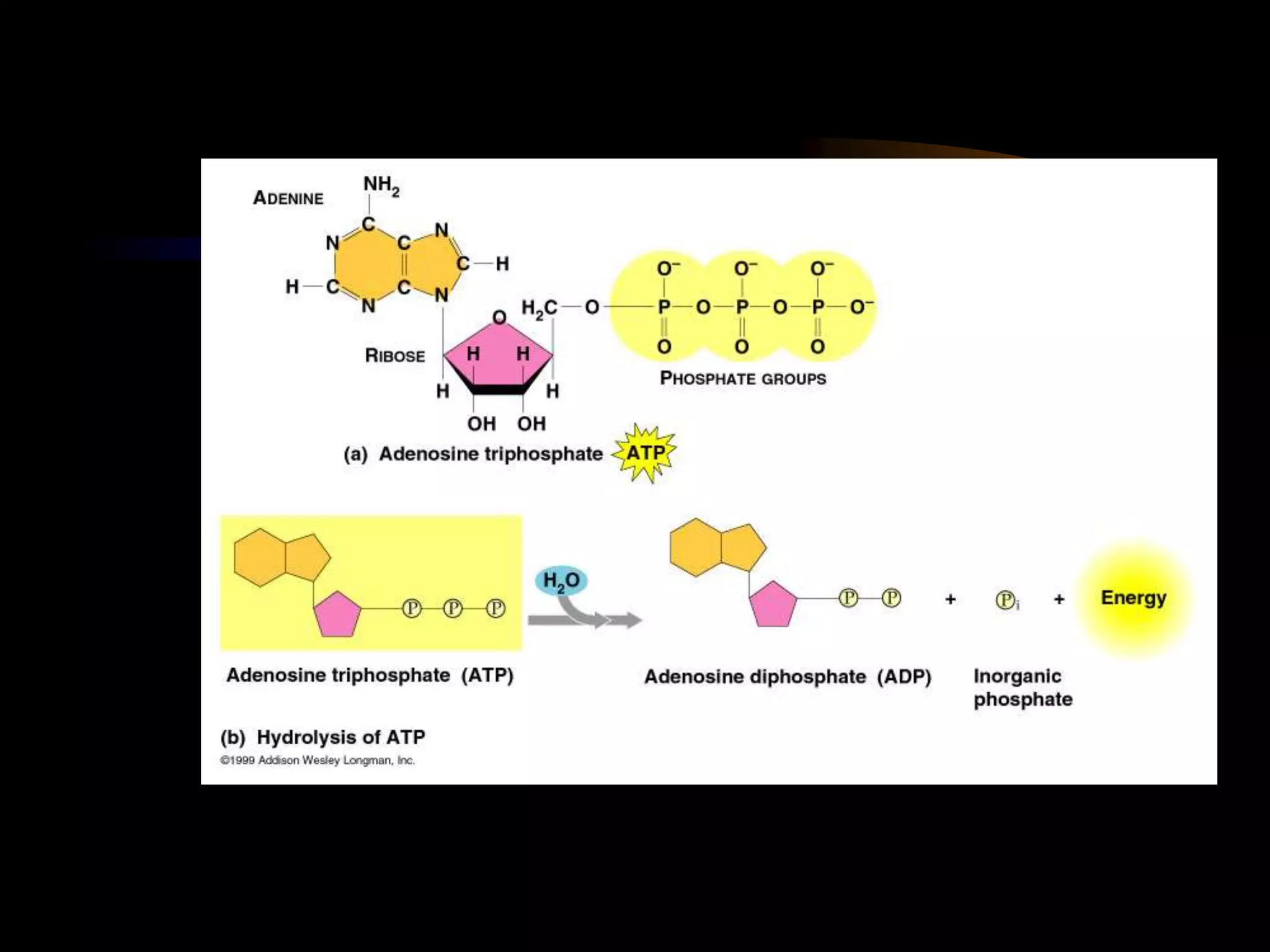
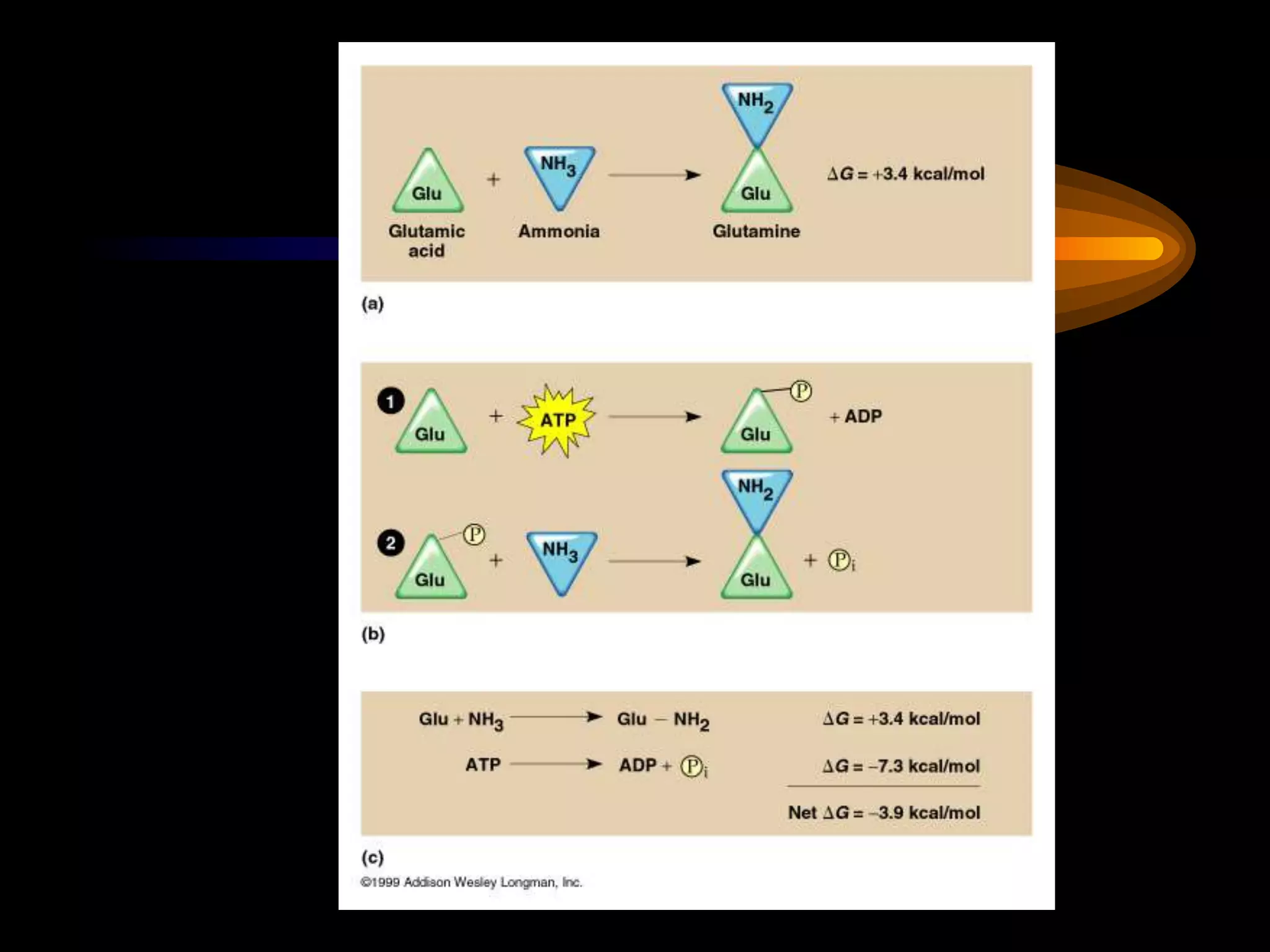
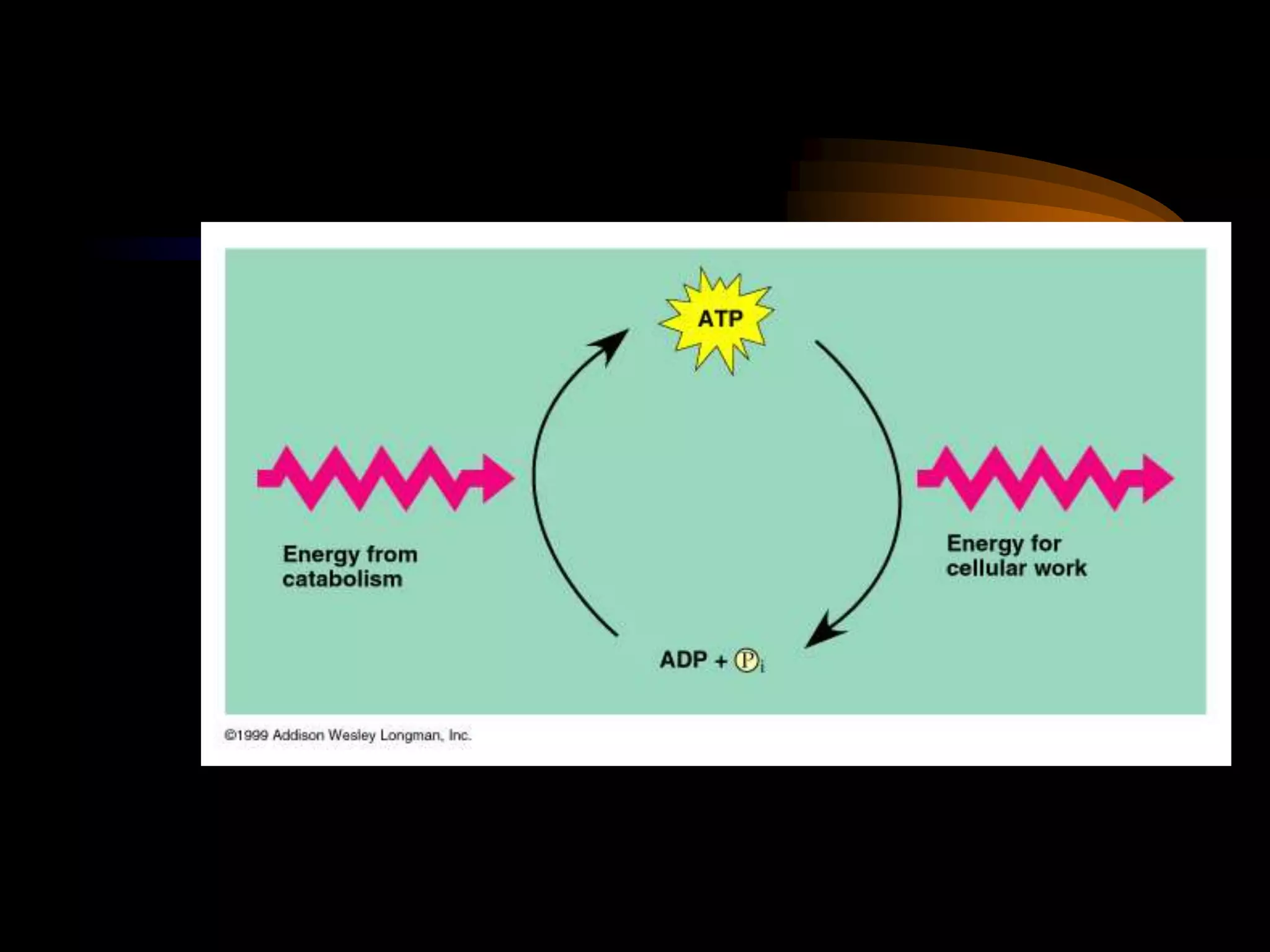
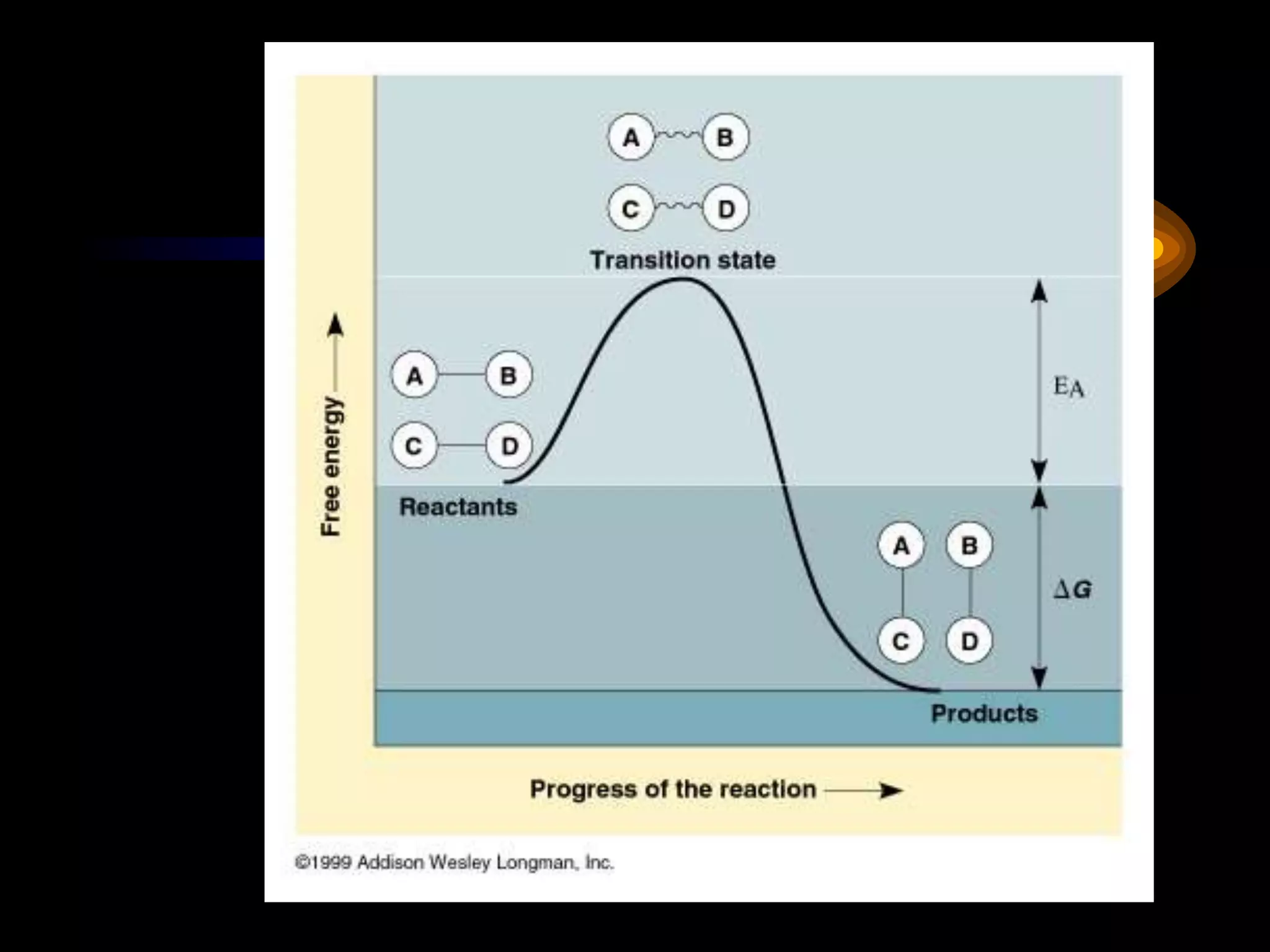
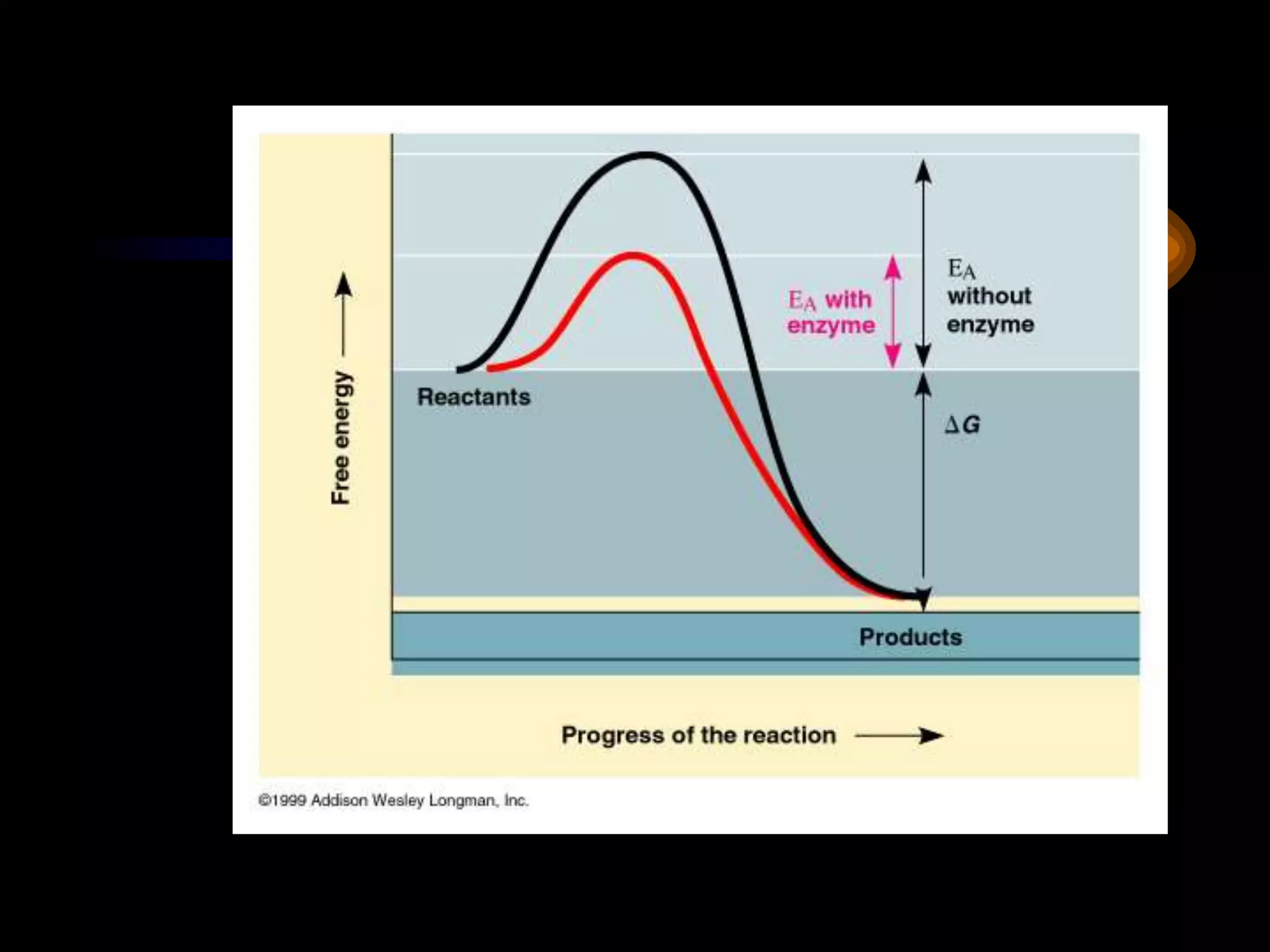
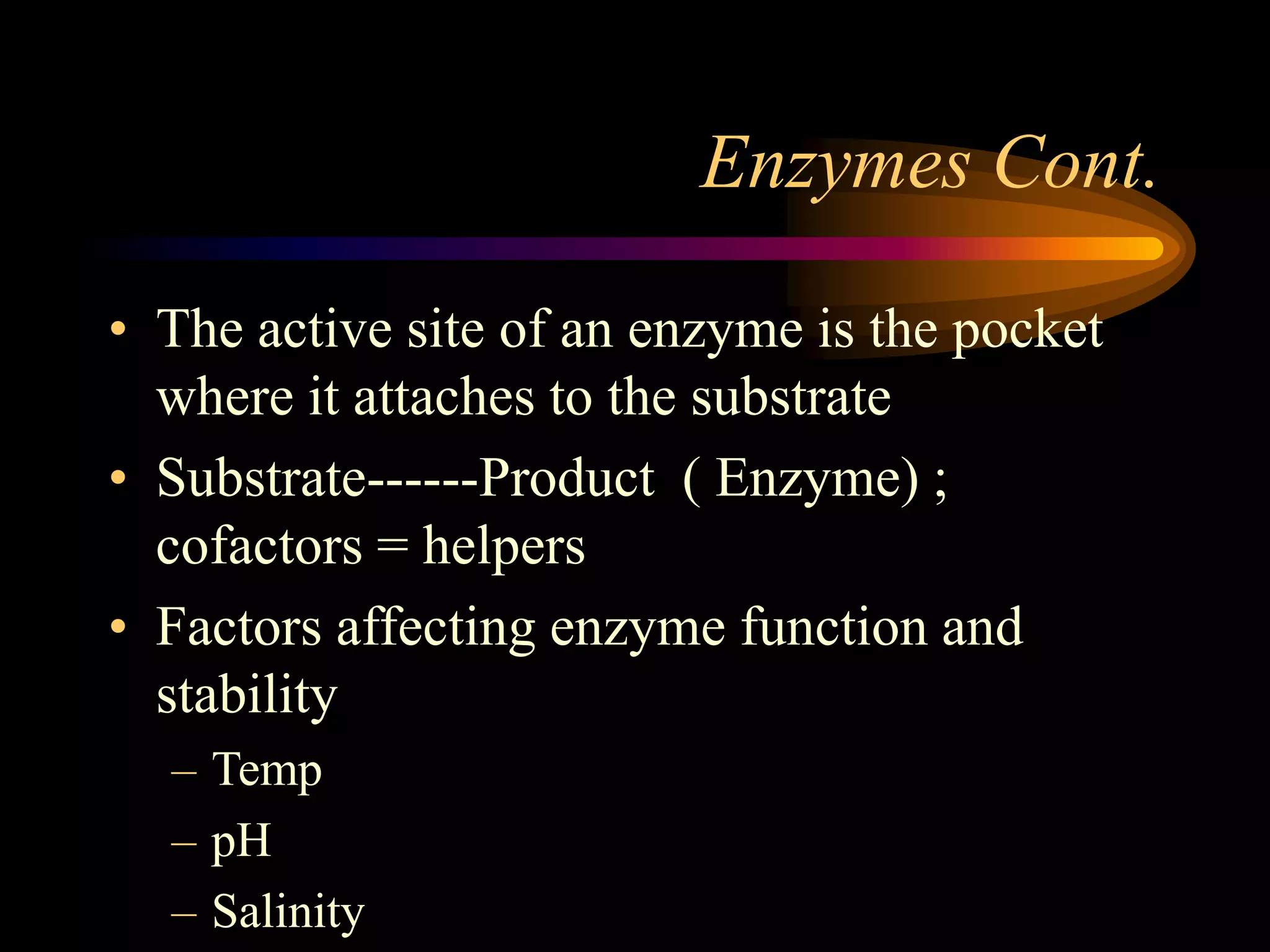
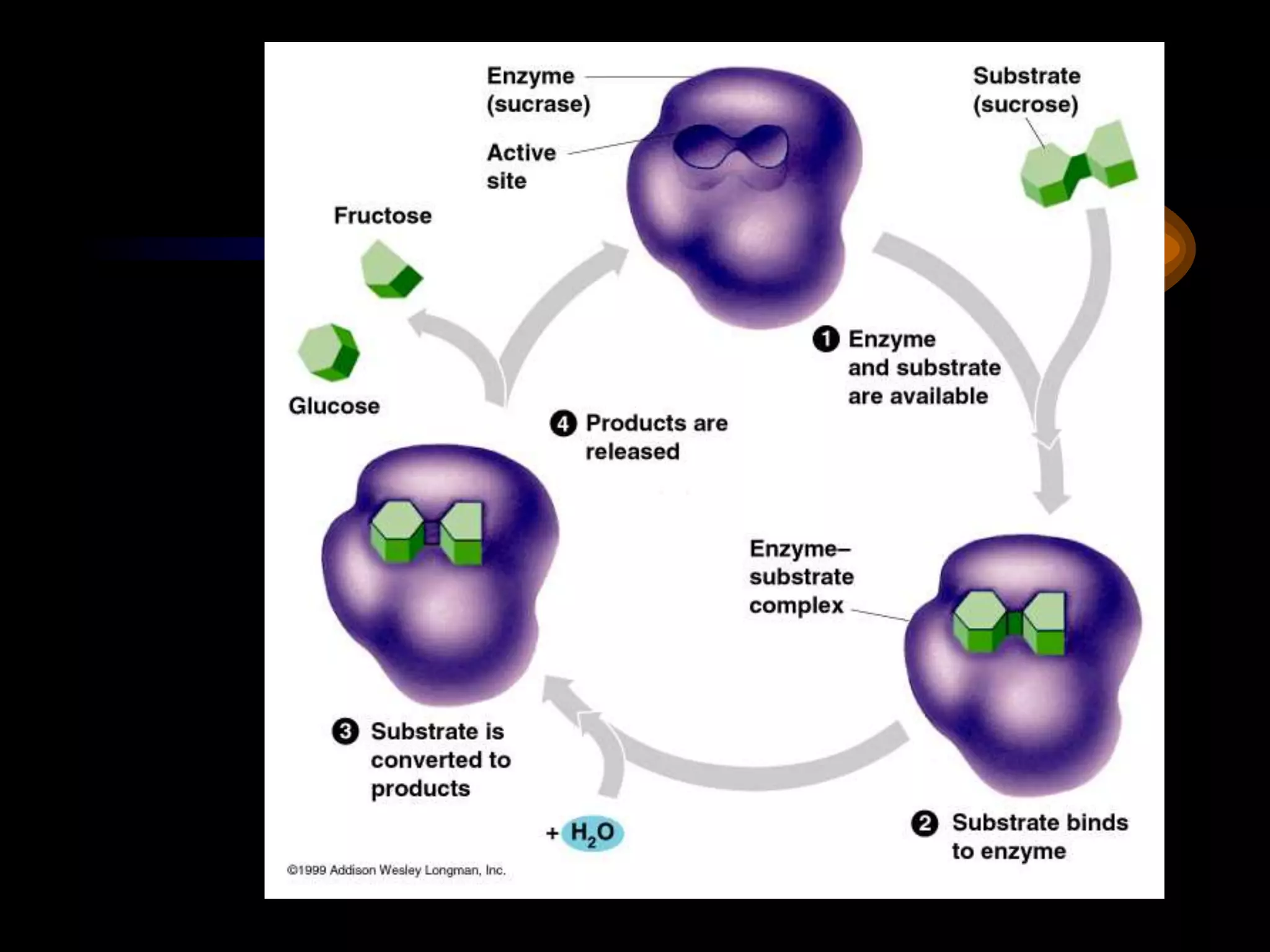
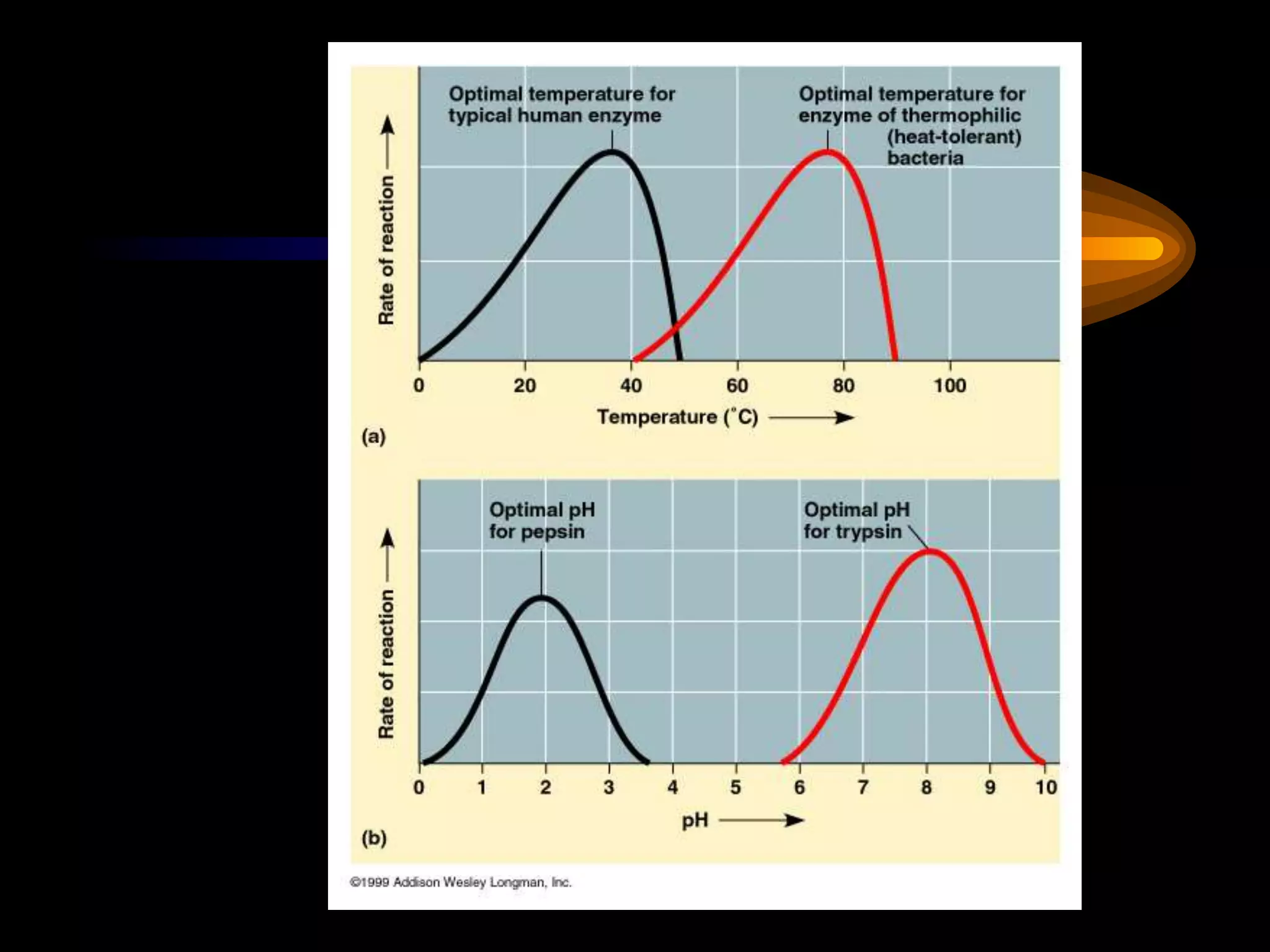
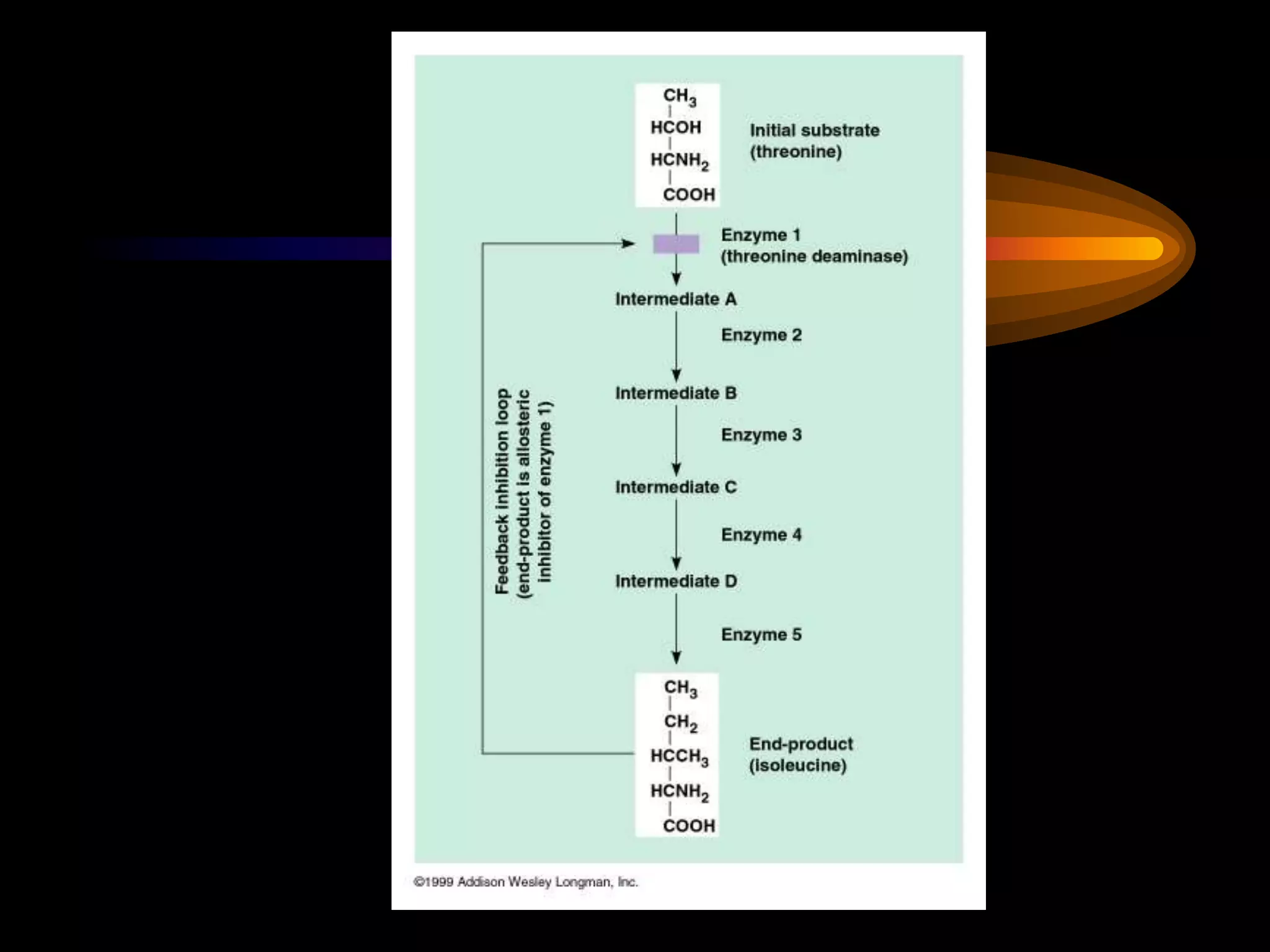
Metabolism refers to the chemical processes that take place inside cells, including thousands of reactions that build up or break down complex compounds. These anabolic and catabolic pathways are concerned with managing the cell's energy and resources. Metabolism involves the conversion of energy from one form to another, as governed by the laws of thermodynamics. ATP acts as the main energy currency, using energy released from its phosphate bonds to power cellular work through exergonic reactions. Enzymes are crucial to metabolism as they catalyze reactions and allow them to proceed faster by lowering their activation energy. Metabolic pathways are regulated through feedback inhibition which turns pathways off once a threshold of end products has been reached.