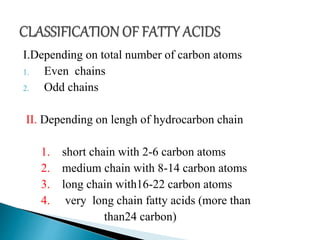
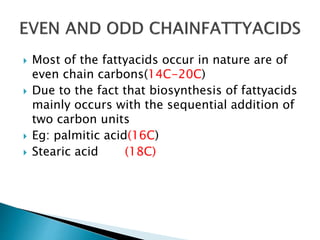
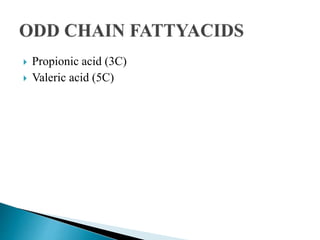
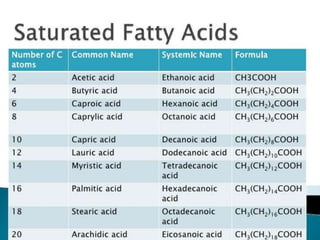
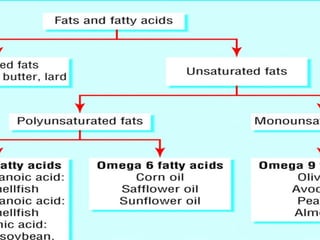
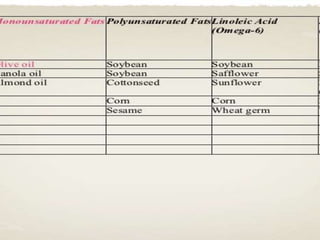
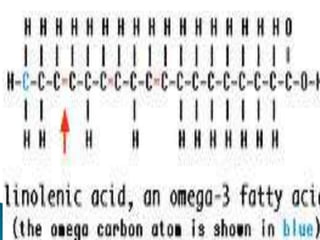
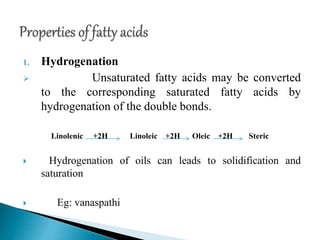
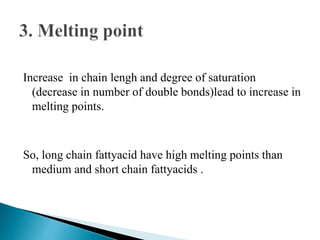
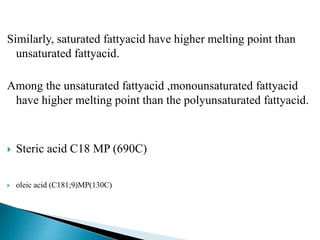
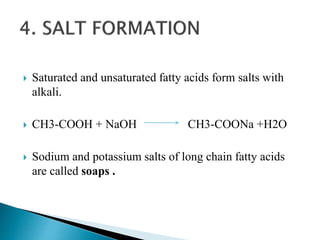
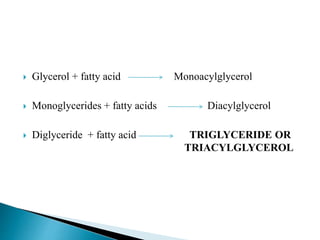
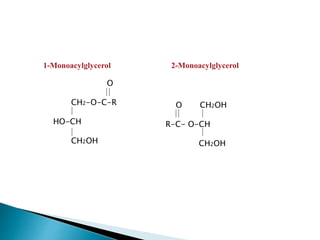
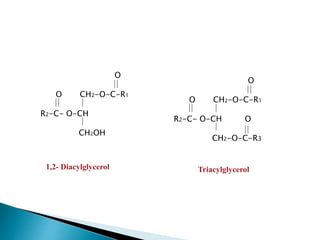
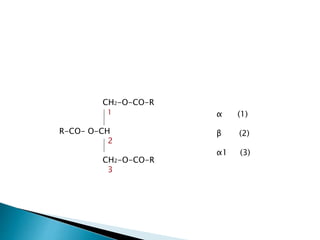
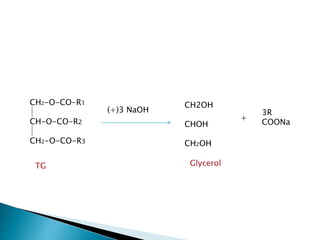
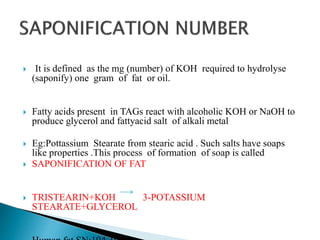
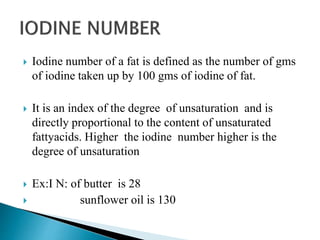
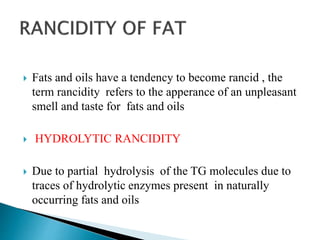
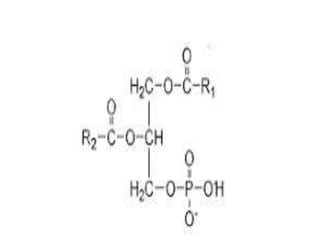
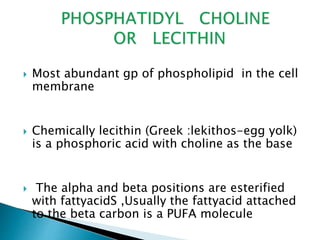
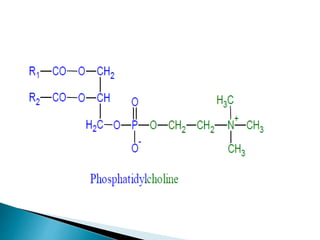
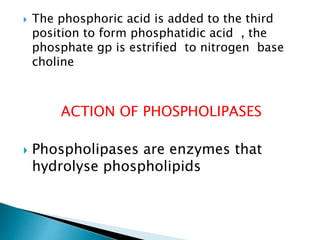
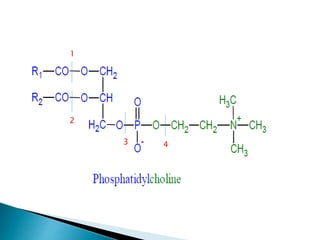
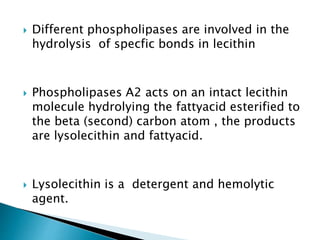
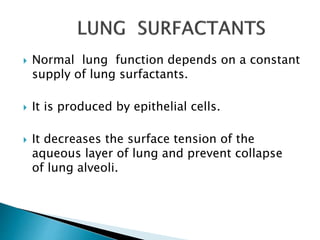
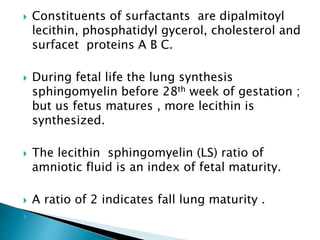
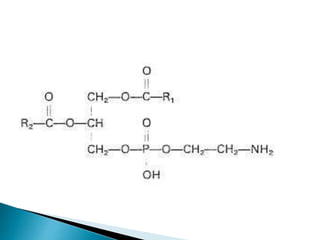
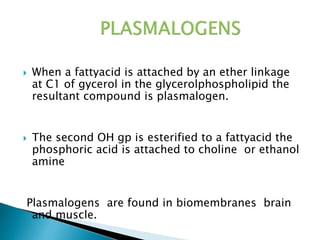
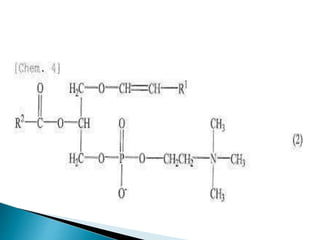
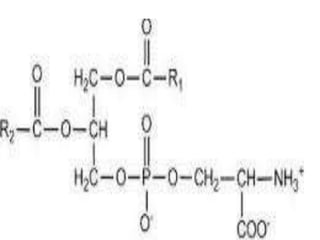
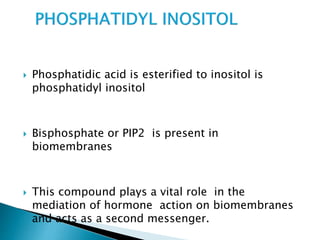
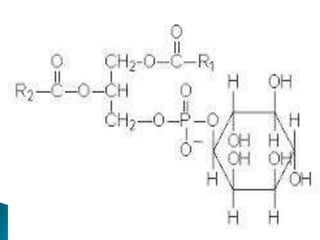
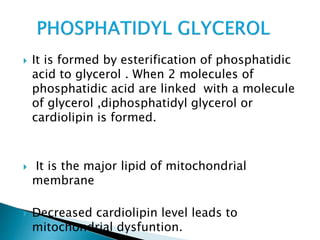
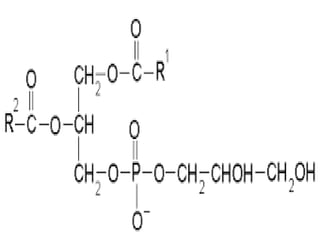
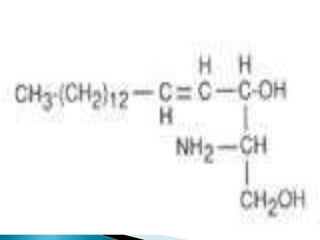
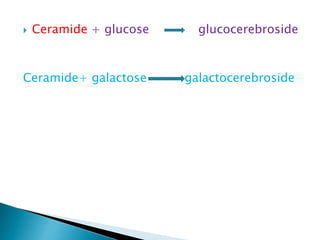
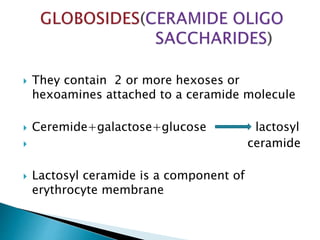
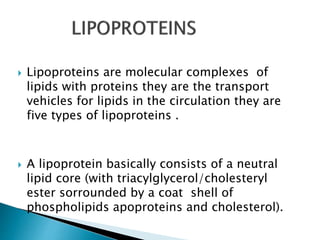
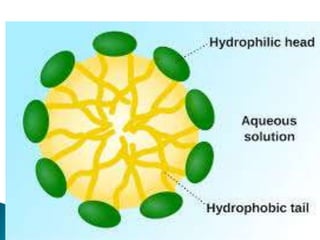
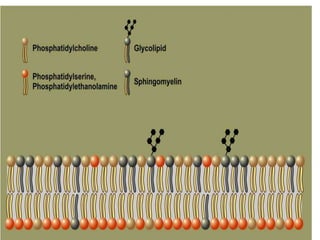
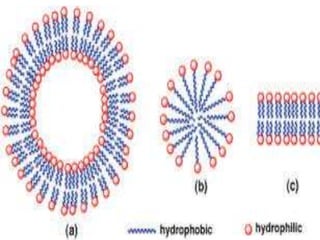
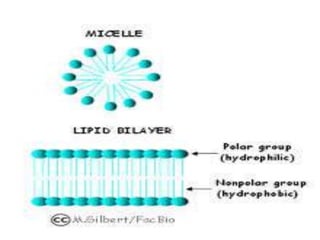
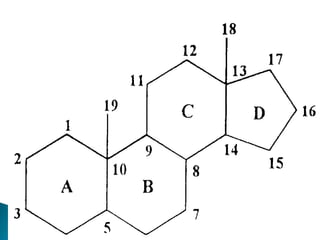
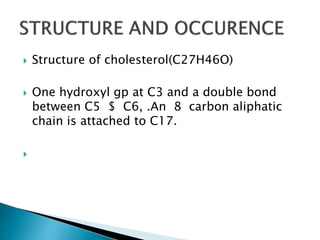
The document discusses lipids and fatty acids. It defines lipids as organic substances that are insoluble in water but soluble in organic solvents. Lipids are classified into simple, compound, derived and miscellaneous lipids. Fatty acids are the most common components of lipids in the body. They are aliphatic carboxylic acids with hydrocarbon side chains. Saturated and unsaturated fatty acids occur naturally. Phospholipids are an important class of compound lipids that form structural components of biological membranes and are involved in various metabolic functions. The most abundant phospholipid is lecithin, which contains phosphoric acid esterified to glycerol and choline.