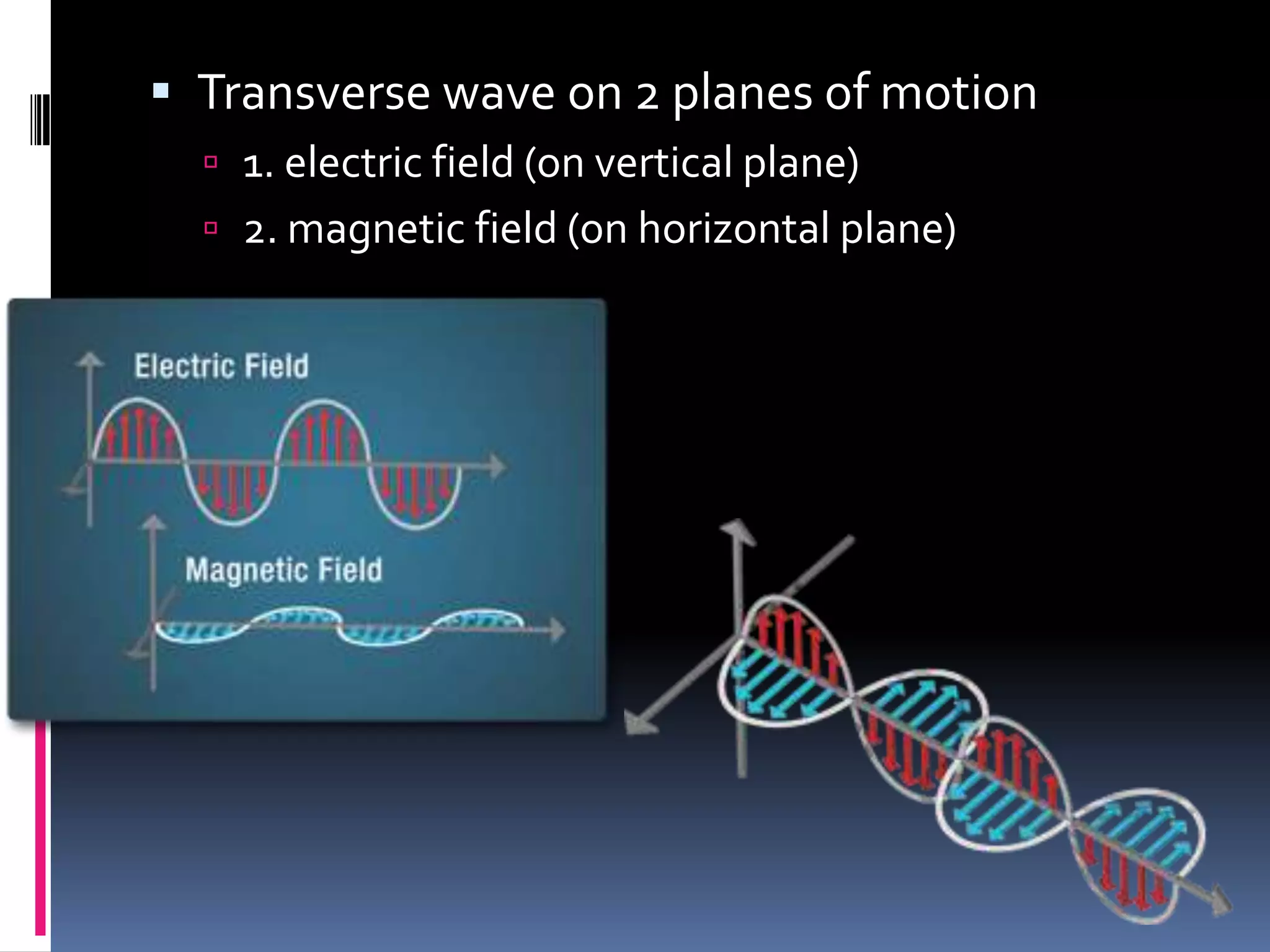
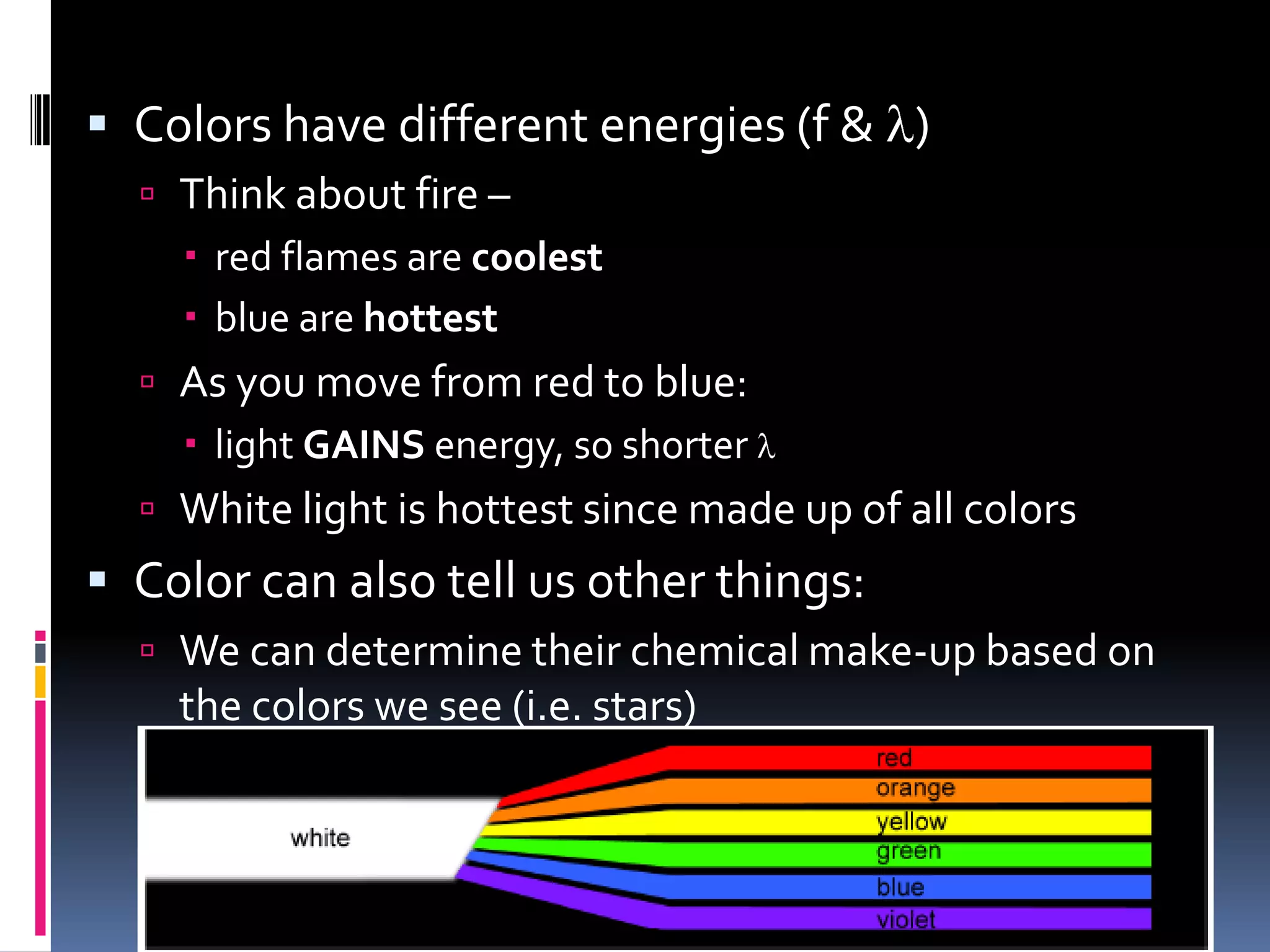
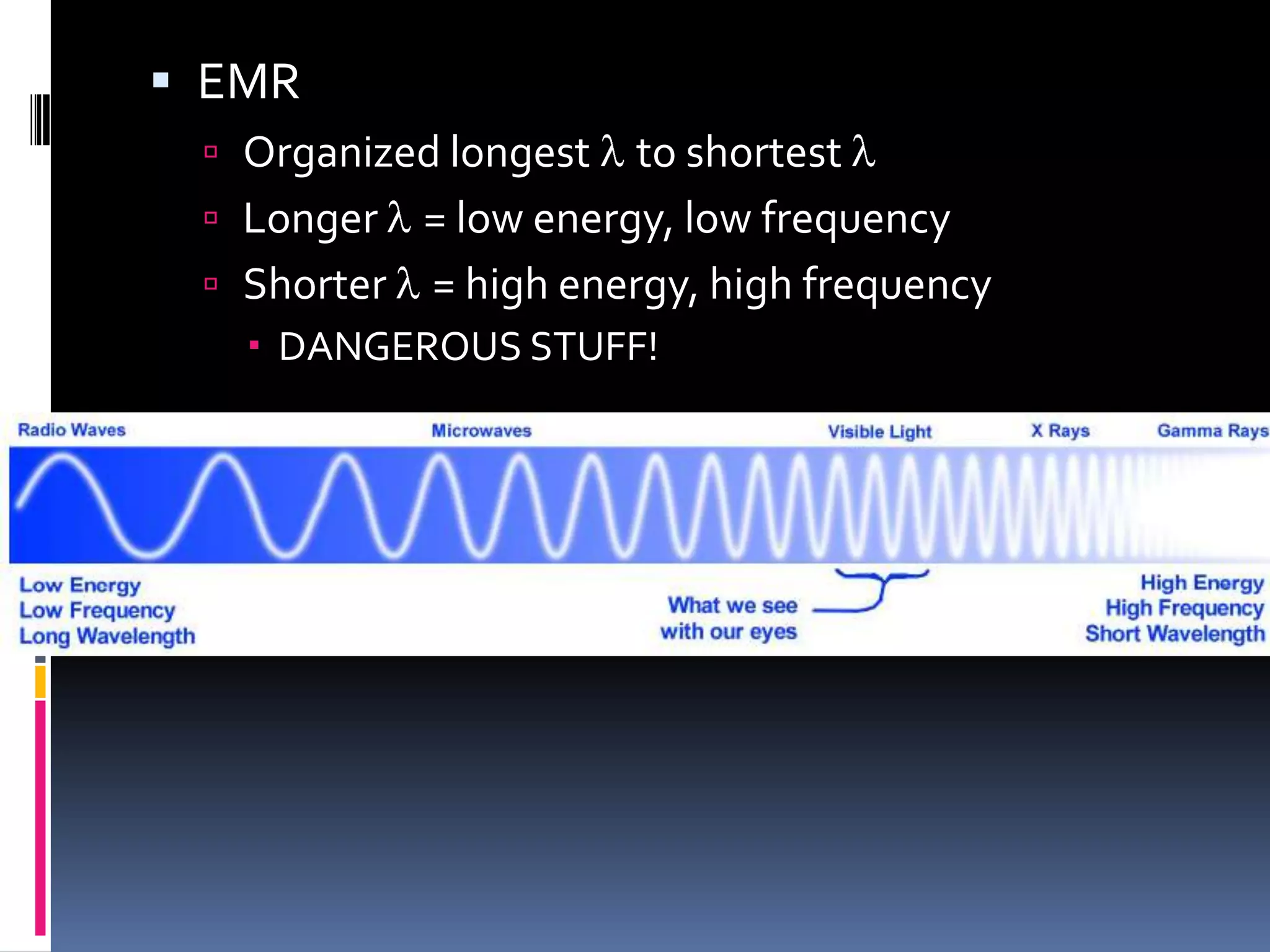
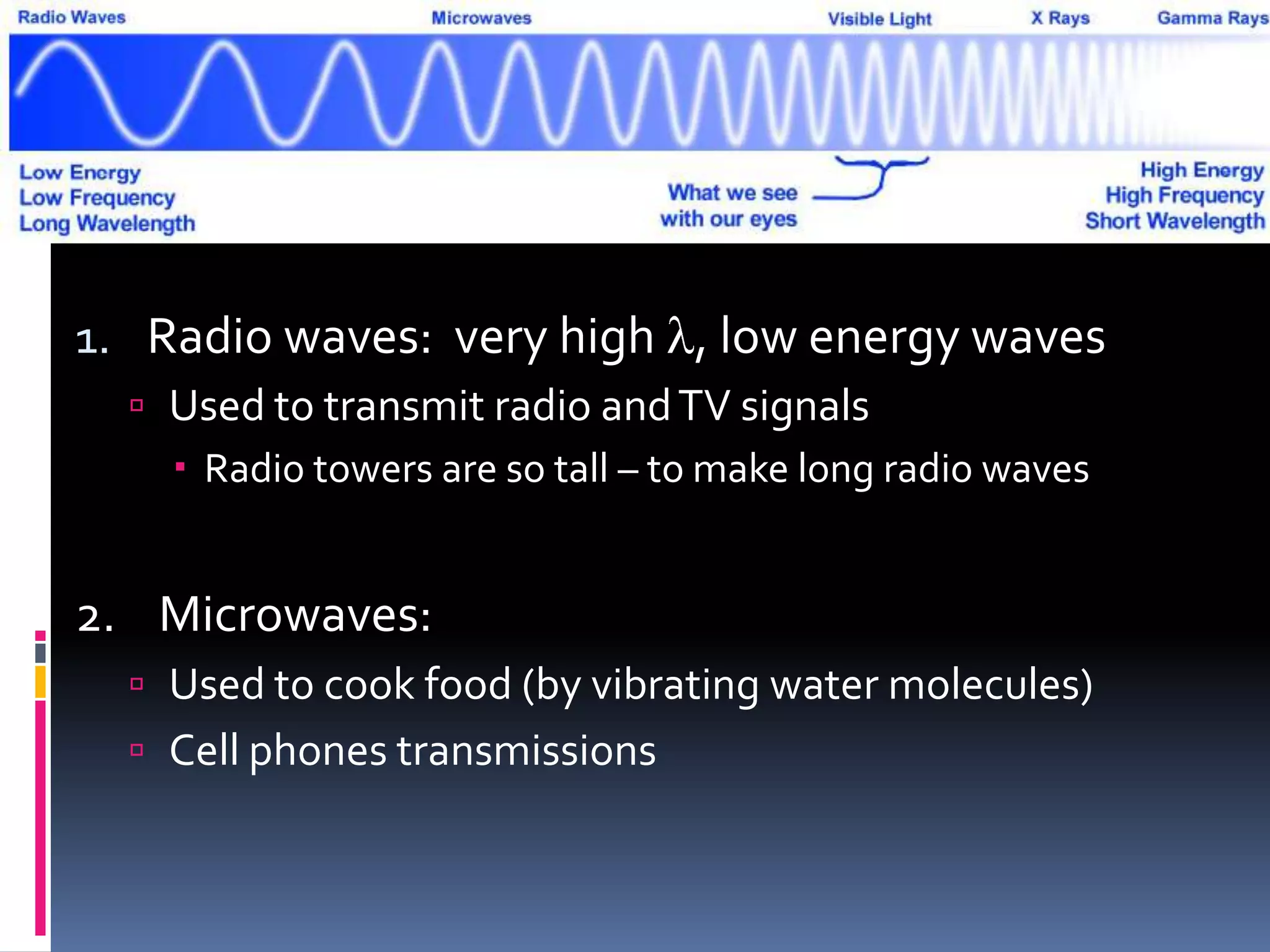
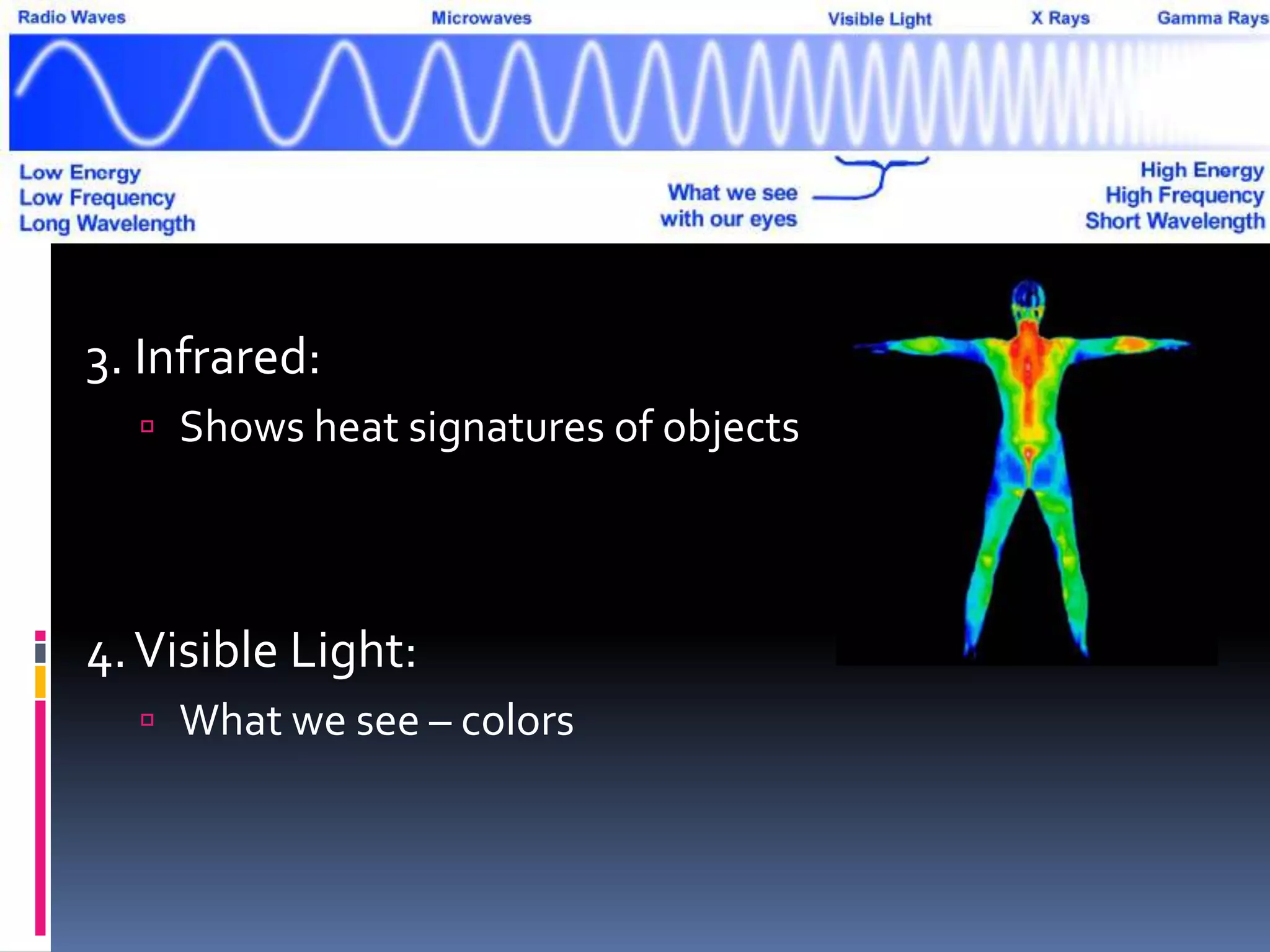
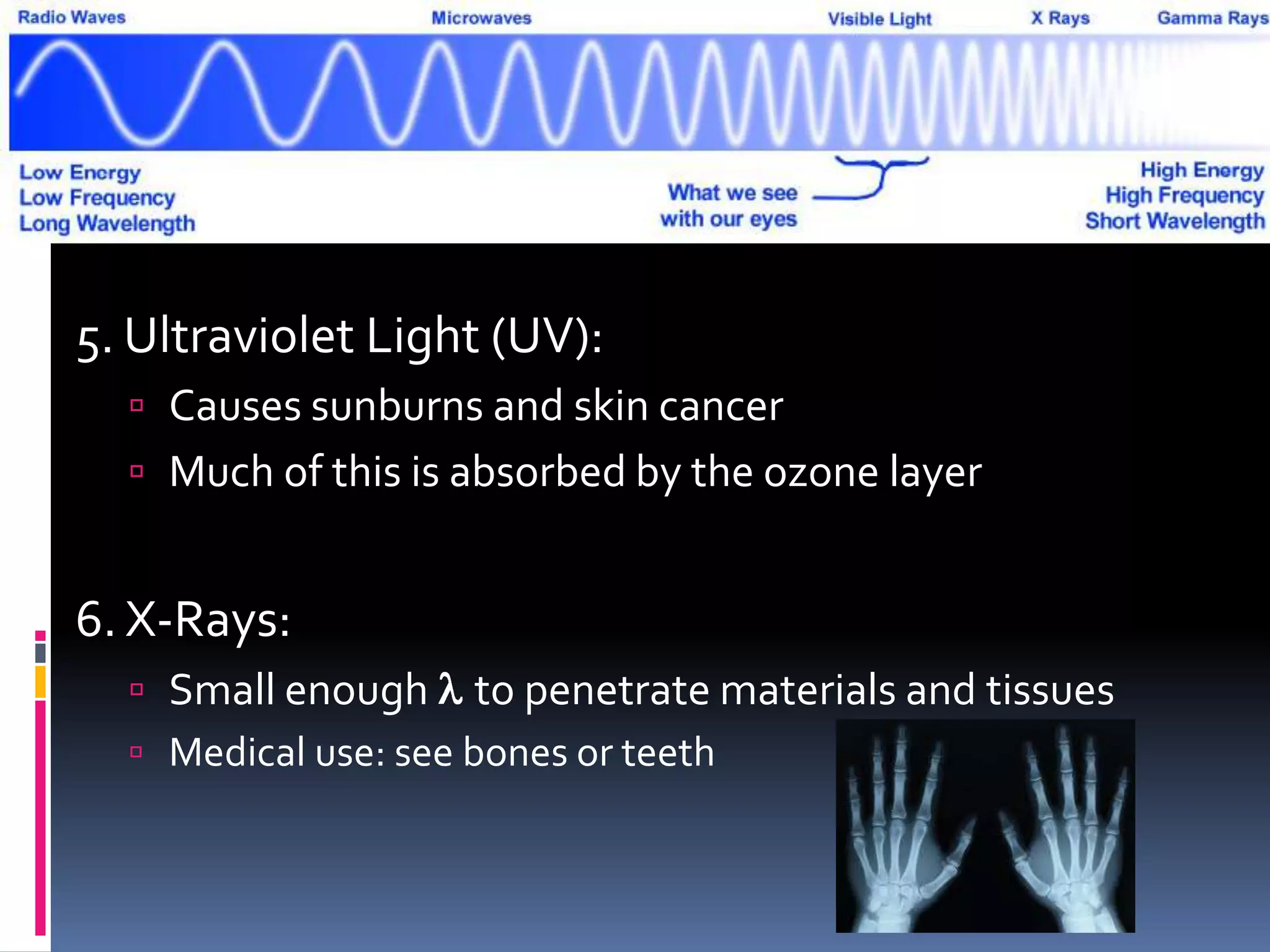
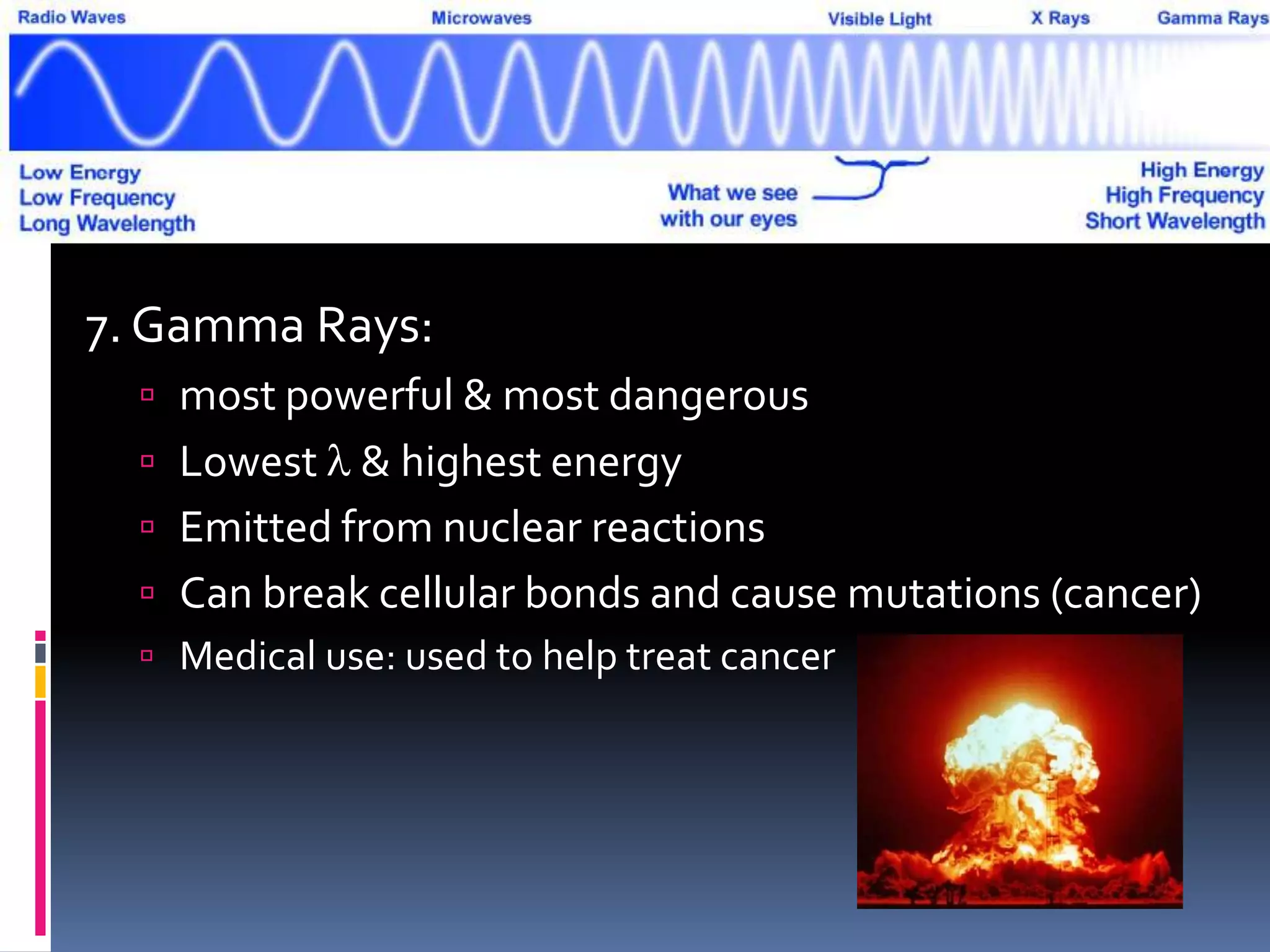
Light can act as both a wave and particle. It is made up of photons that travel in transverse waves. Light is produced when electrons in atoms gain and lose energy by moving closer to or farther from the nucleus. Different light sources like incandescent bulbs, fluorescent bulbs, and LEDs produce light through various electron energy transfer processes. Light travels at the universal speed limit of about 300 million meters per second. Only a small portion of the electromagnetic spectrum, called visible light, is visible to the human eye.