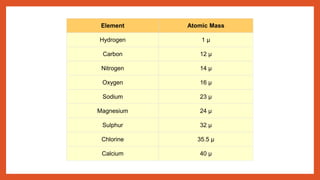
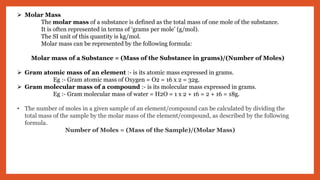
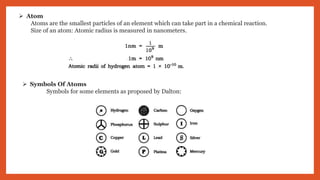
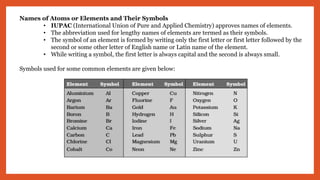
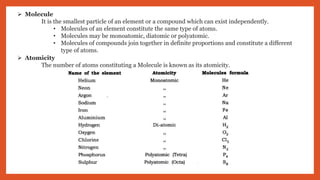
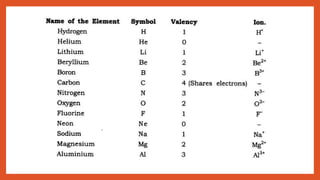
The document outlines the laws of chemical combination, detailing the law of conservation of mass and the law of constant proportions. It explains Dalton's atomic theory, the concepts of atoms, molecules, ions, and valency, along with formulas for calculating atomic and molecular mass and the mole concept. Various chemical formulas and mass calculations provide foundational knowledge in chemistry.



![Some elements show more than one valency, hence termed as variable valency.
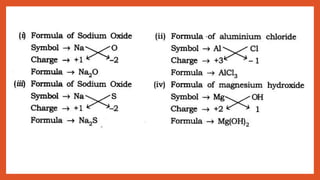
Chemical Formulae
Rules:
1. The valencies or charges on the ion must balance.
2. Metal and non-metal compound should show the name or symbol of the metal first.
e.g., Na+ Cl– → NaCl
3. If a compound consists of polyatomic ions. The ion is enclosed in a bracket before writing the number to
indicate the ratio.
e.g., [SO4]2- → polyatomic radical
H1+ SO4
2- → H2SO4](https://image.slidesharecdn.com/class9atomandmolecules-201011102720/85/Class-9-atom-and-molecules-9-320.jpg)