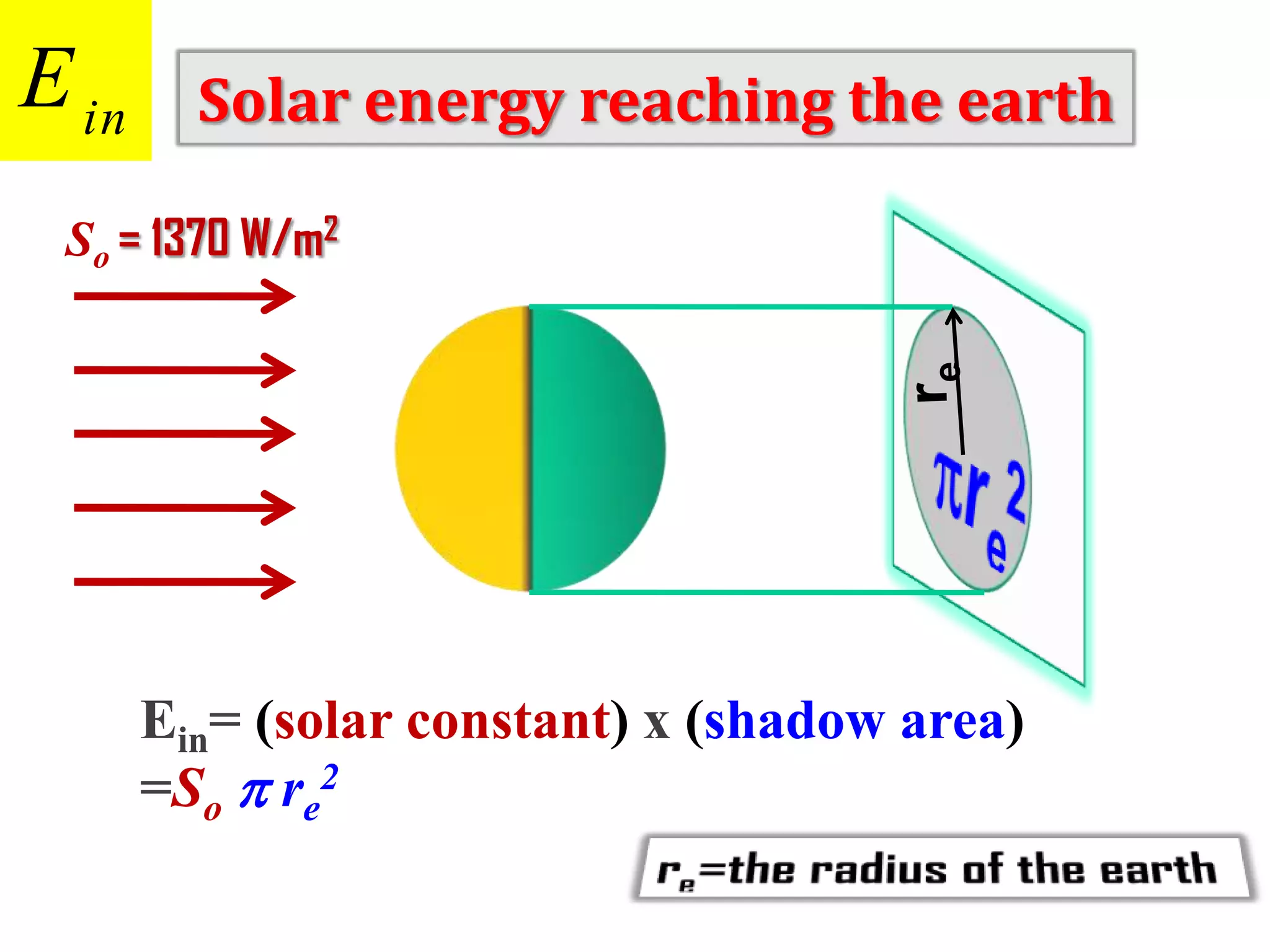
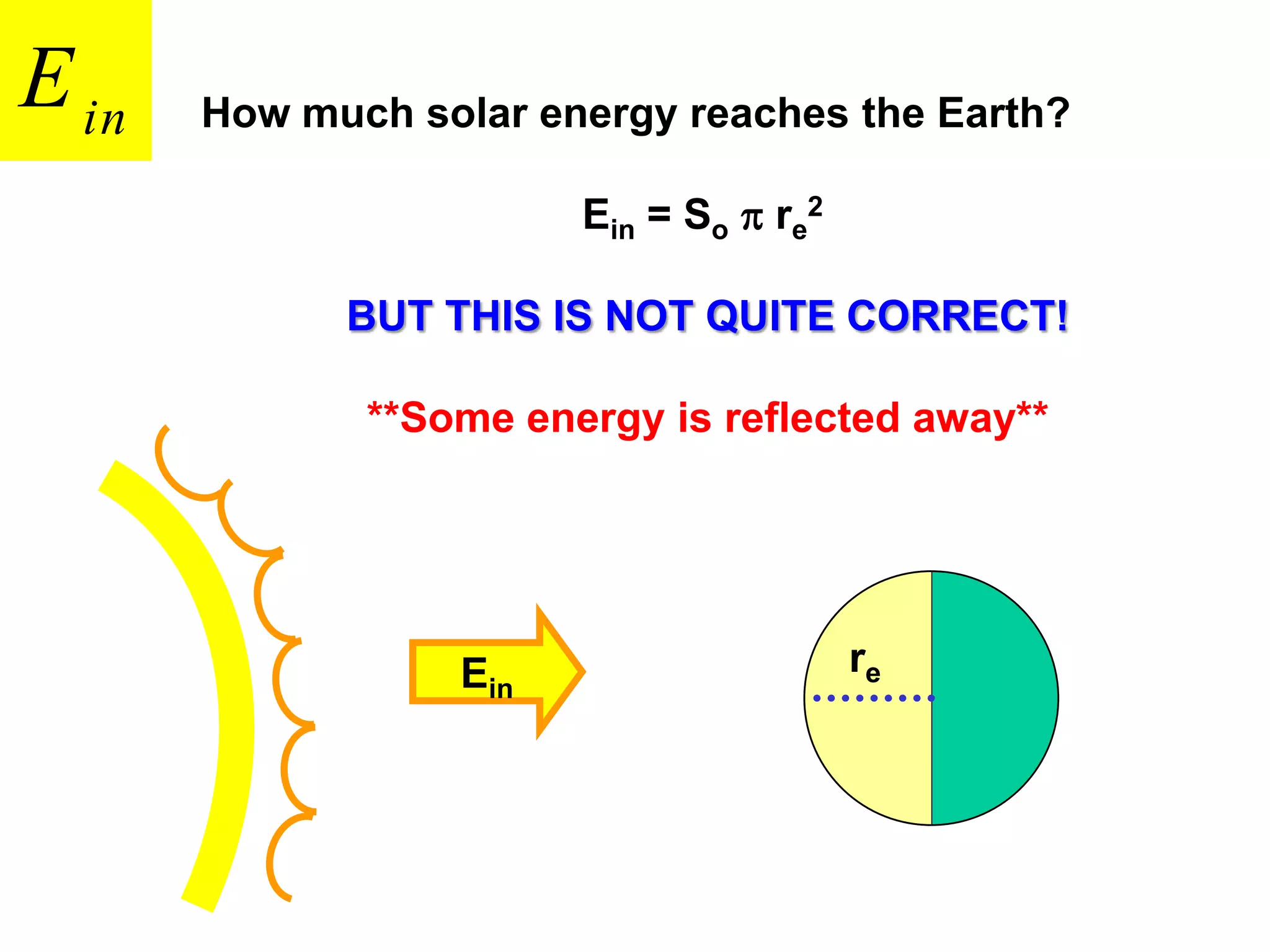
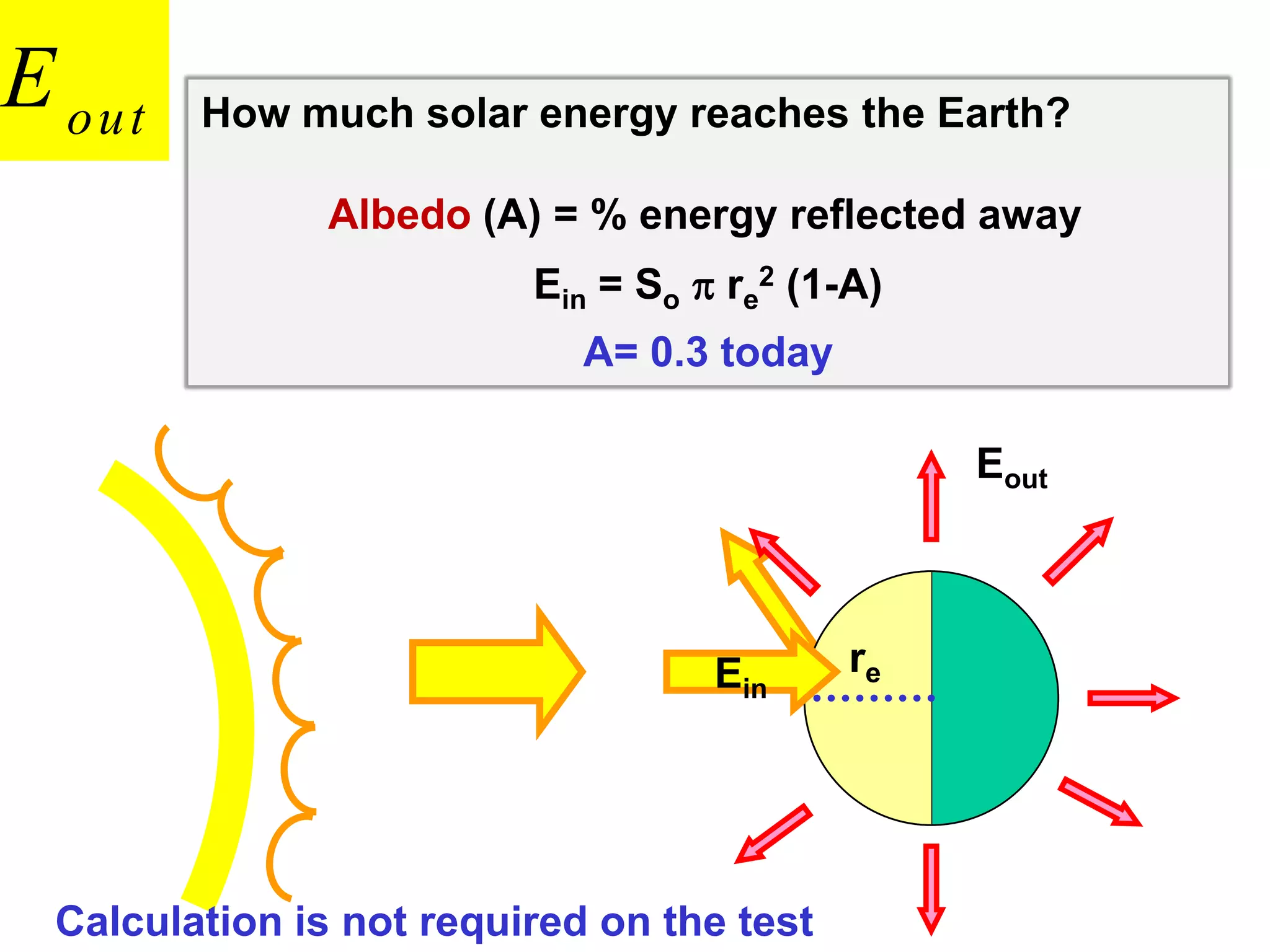
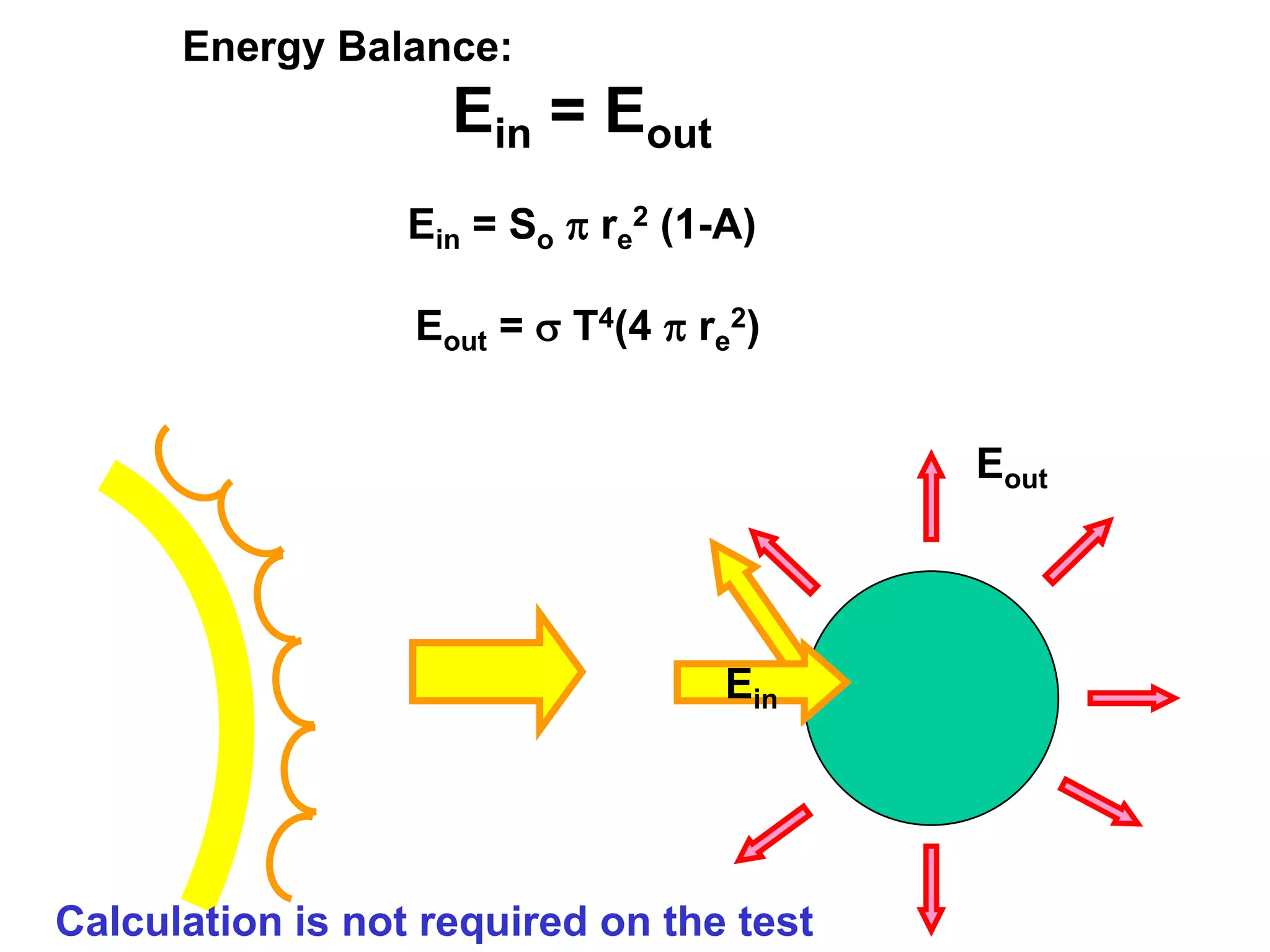
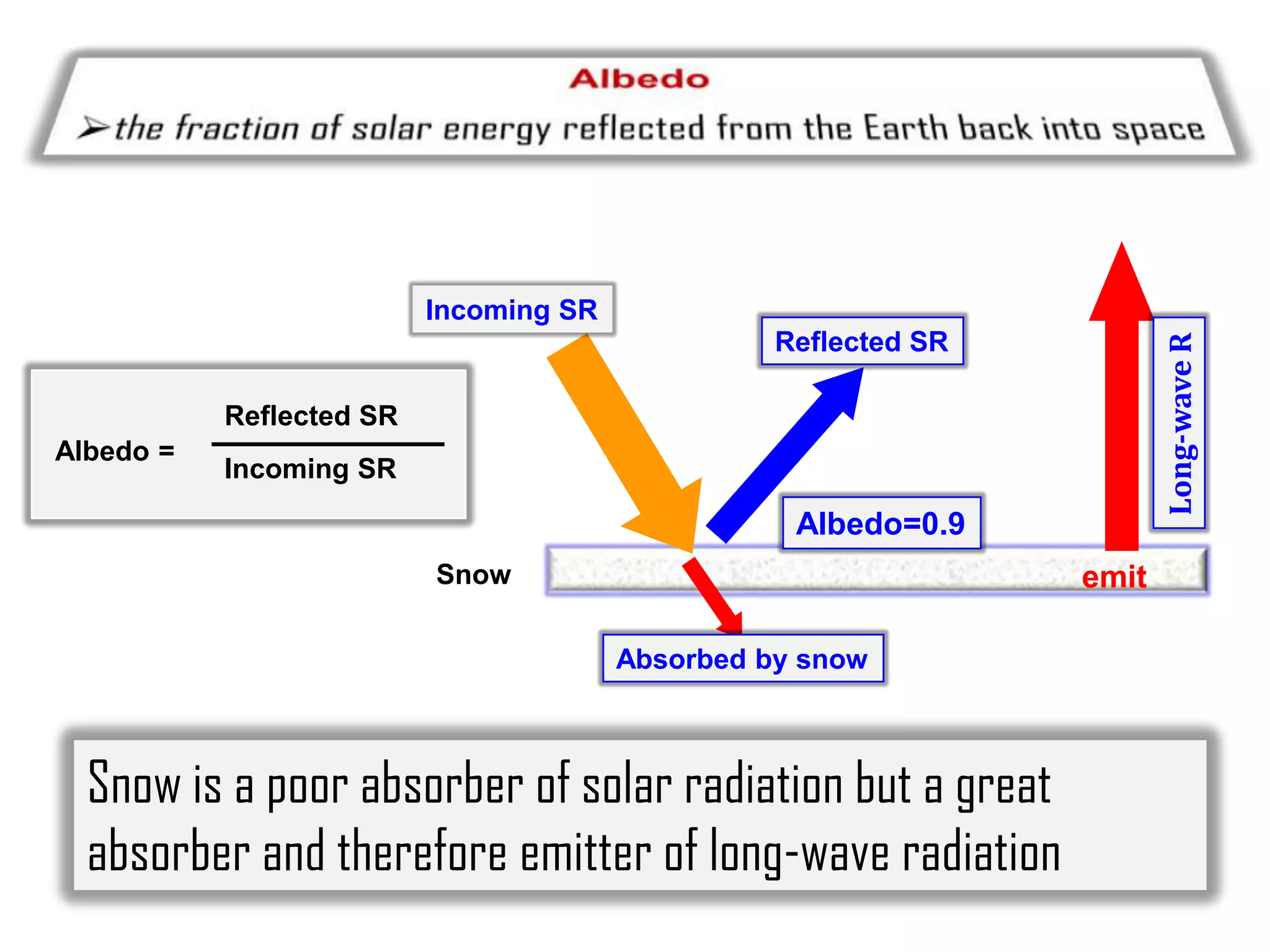
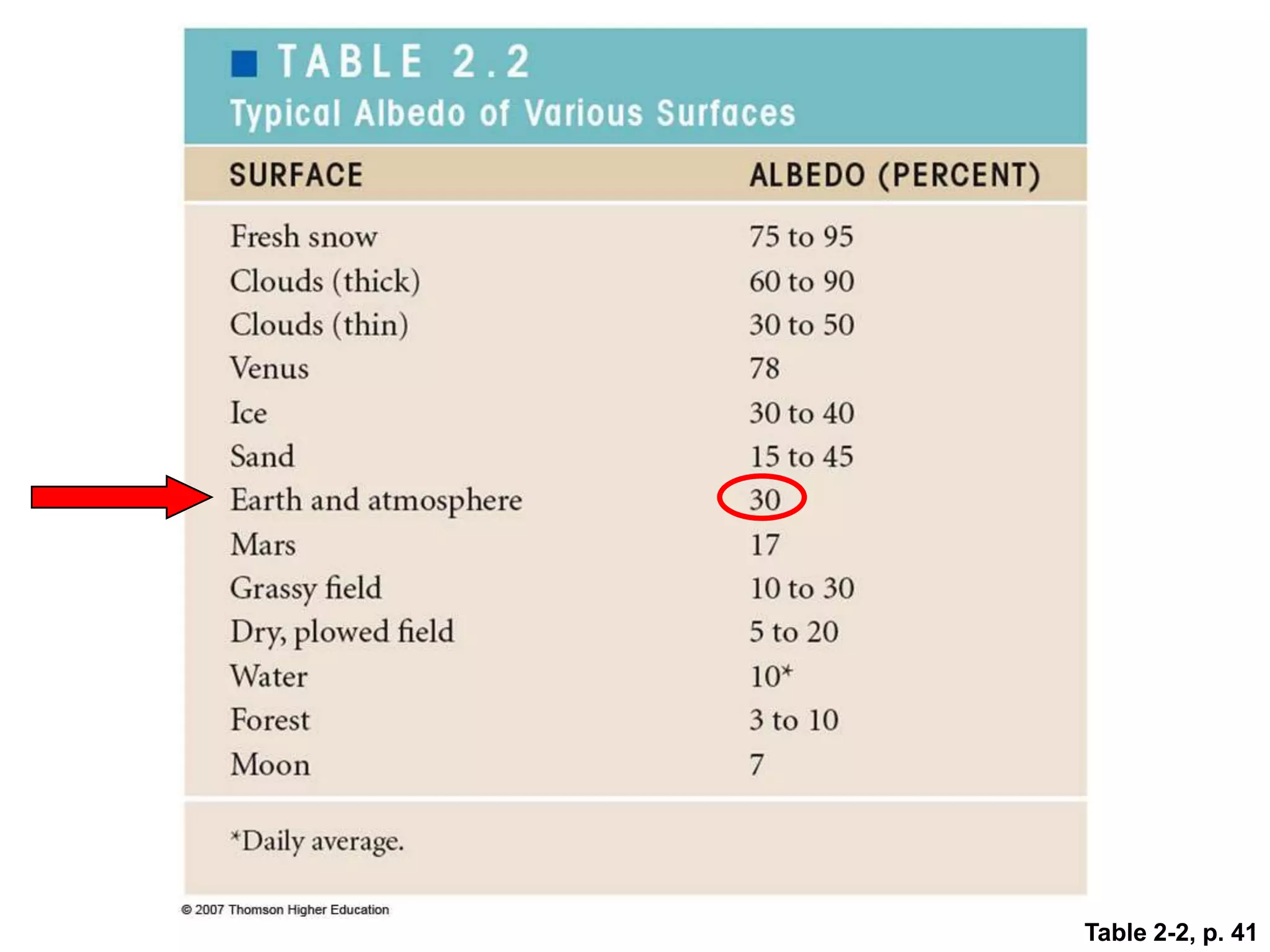
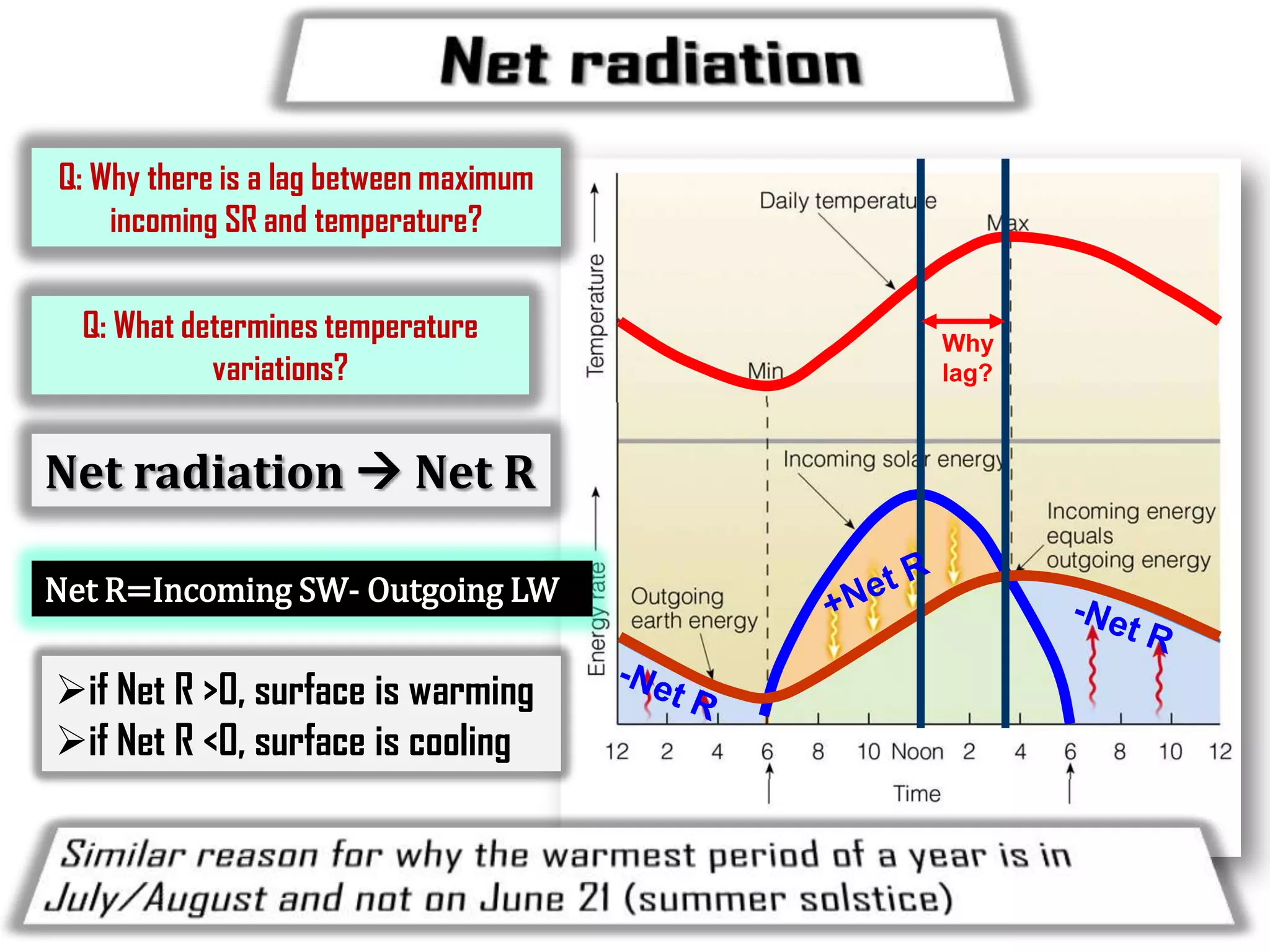
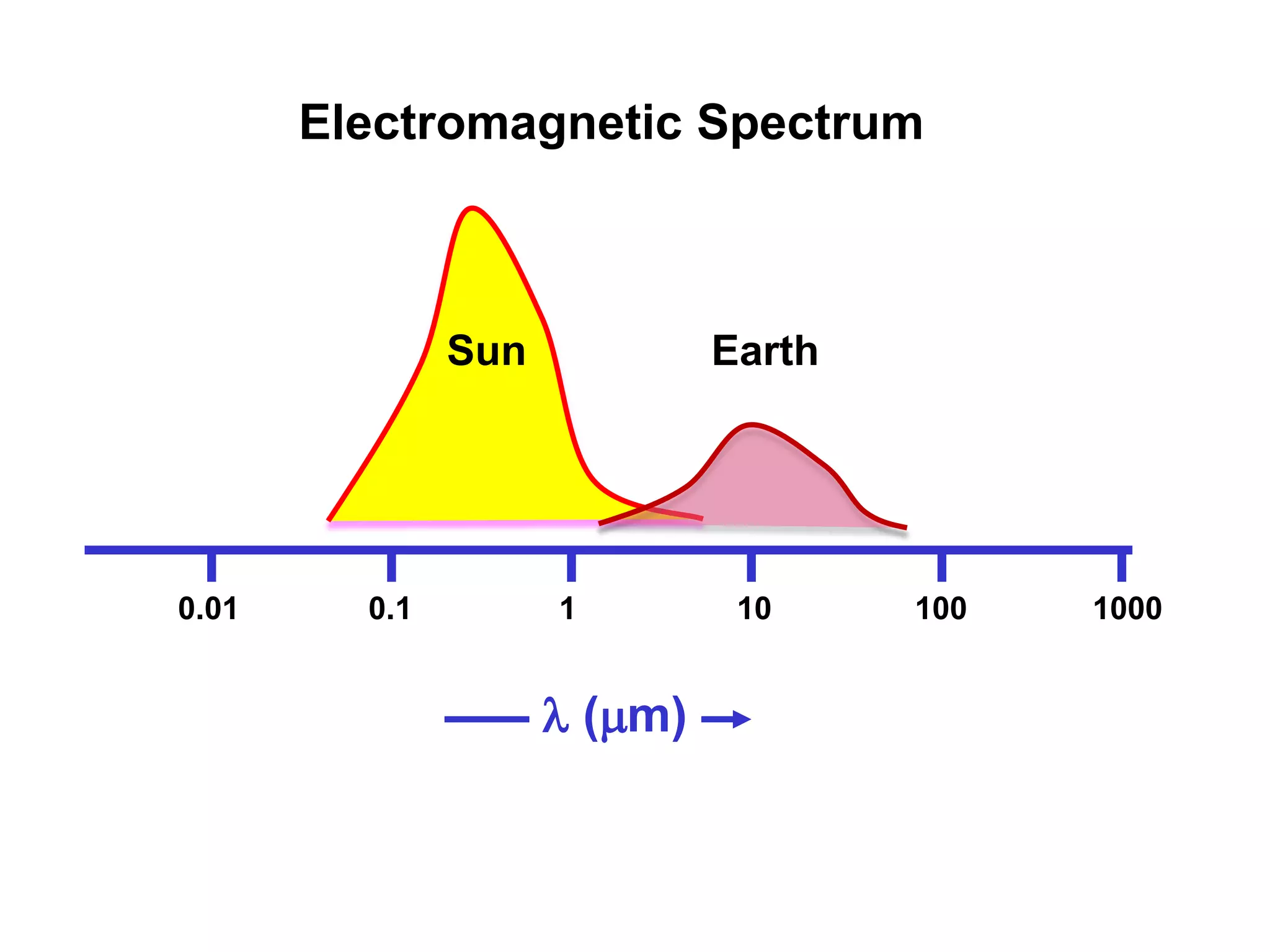
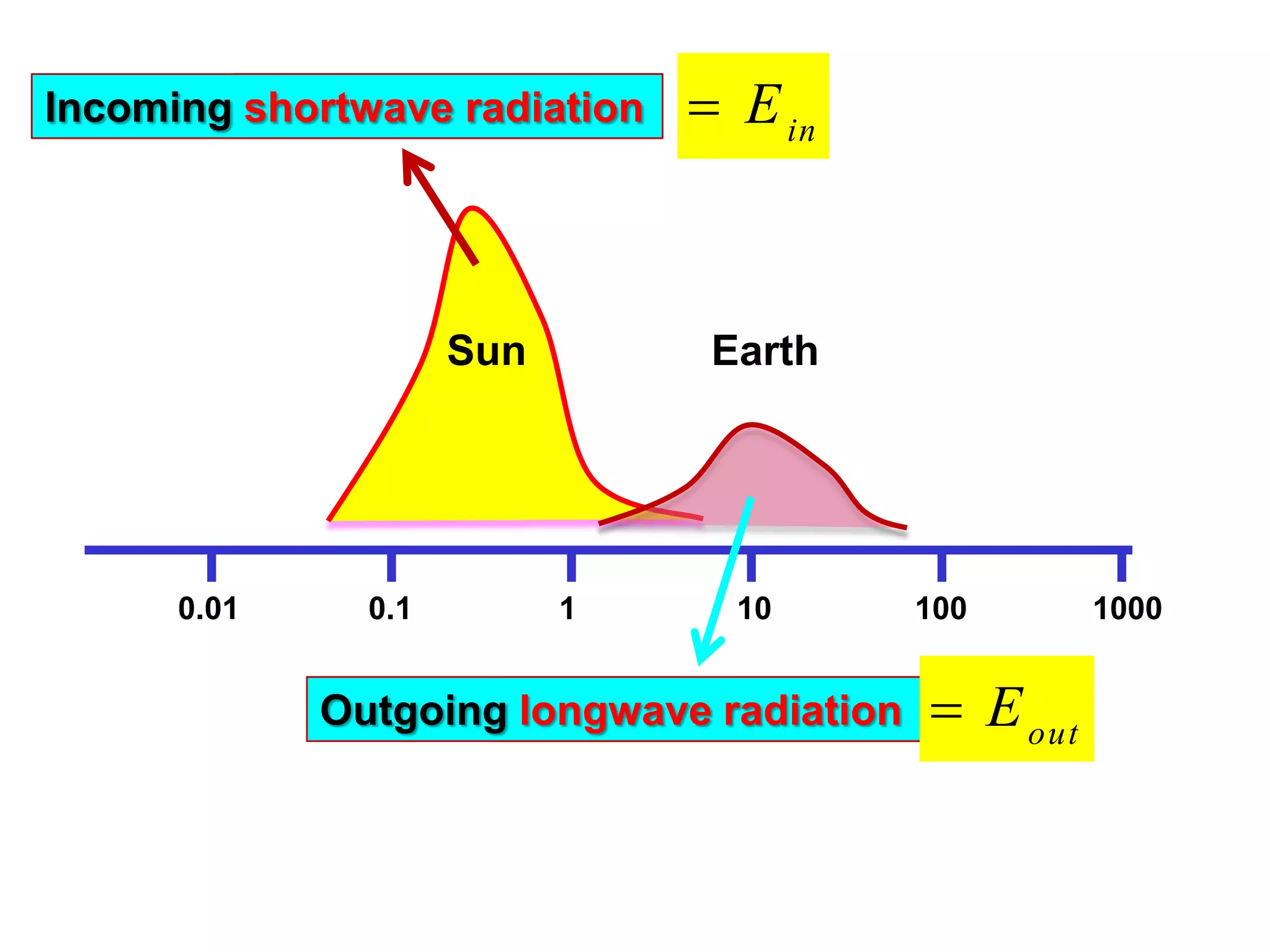
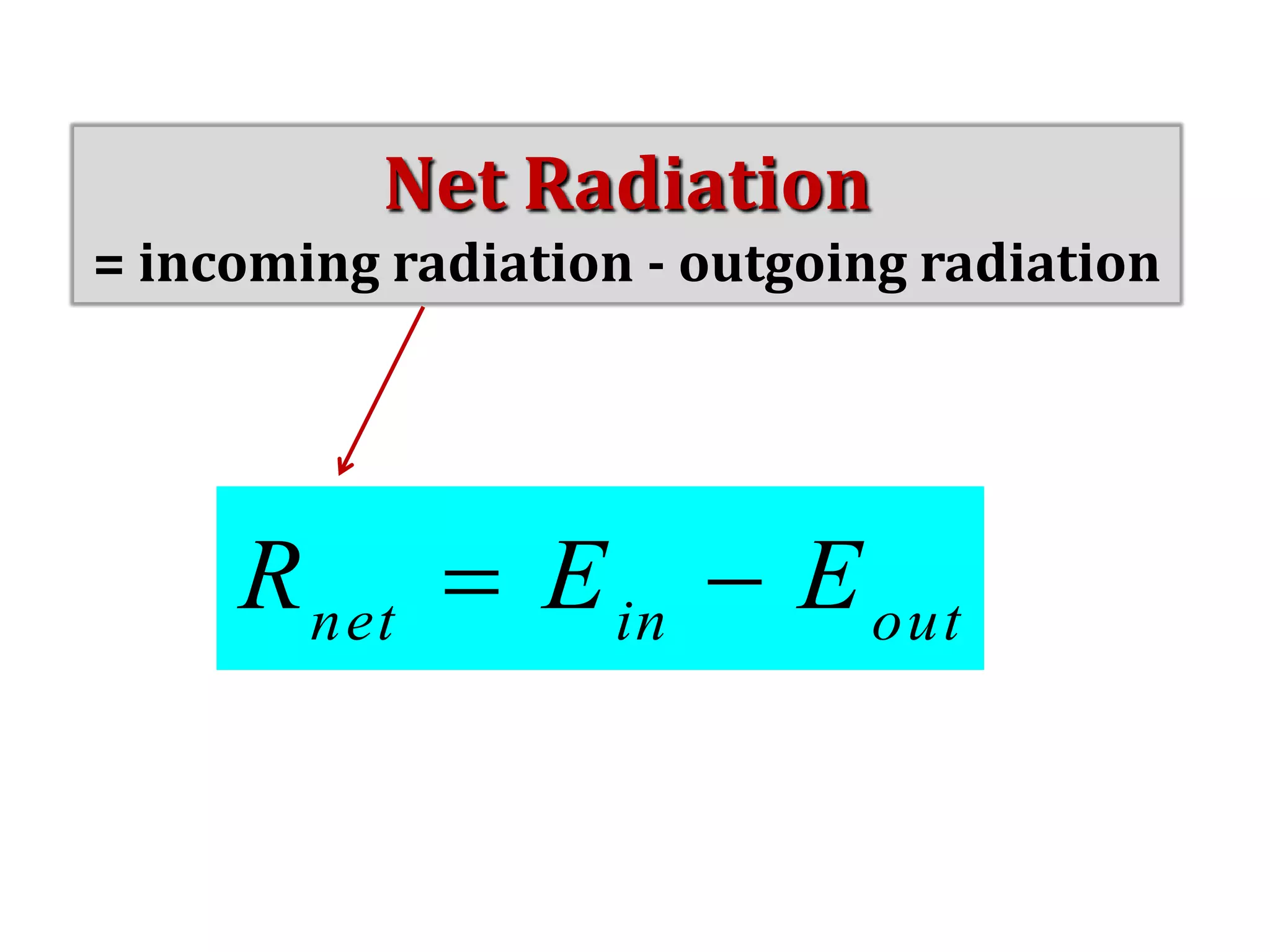
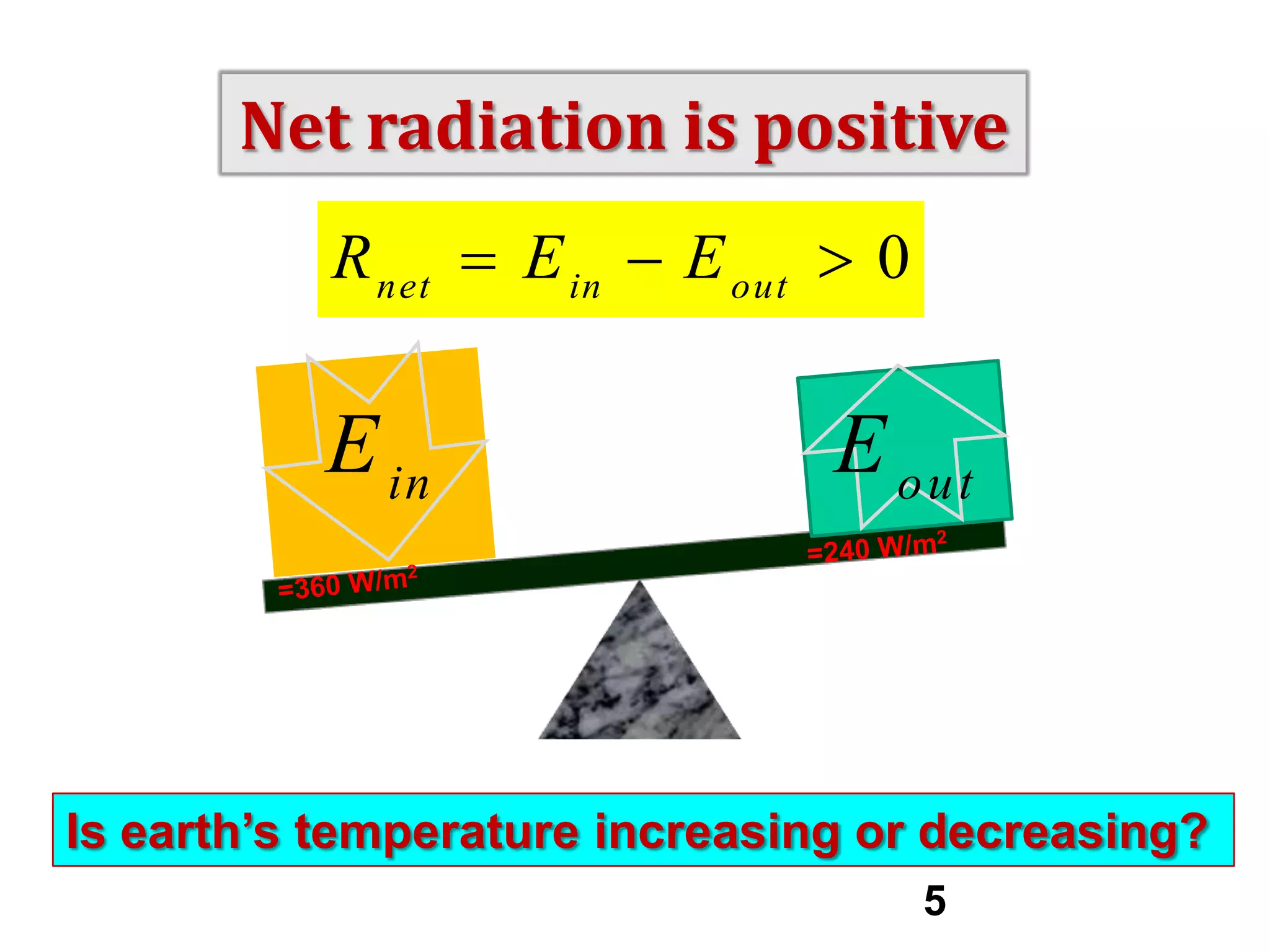
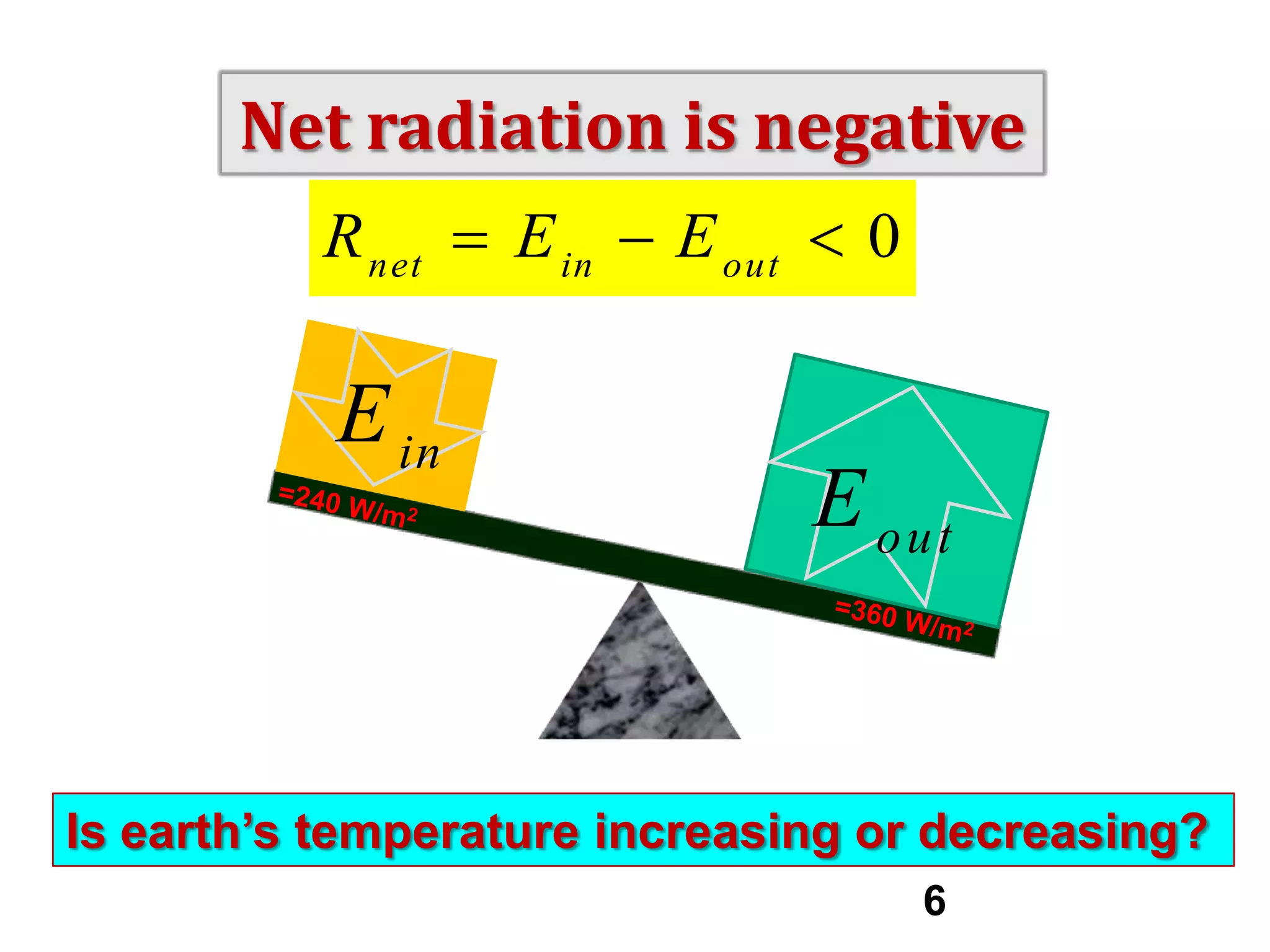
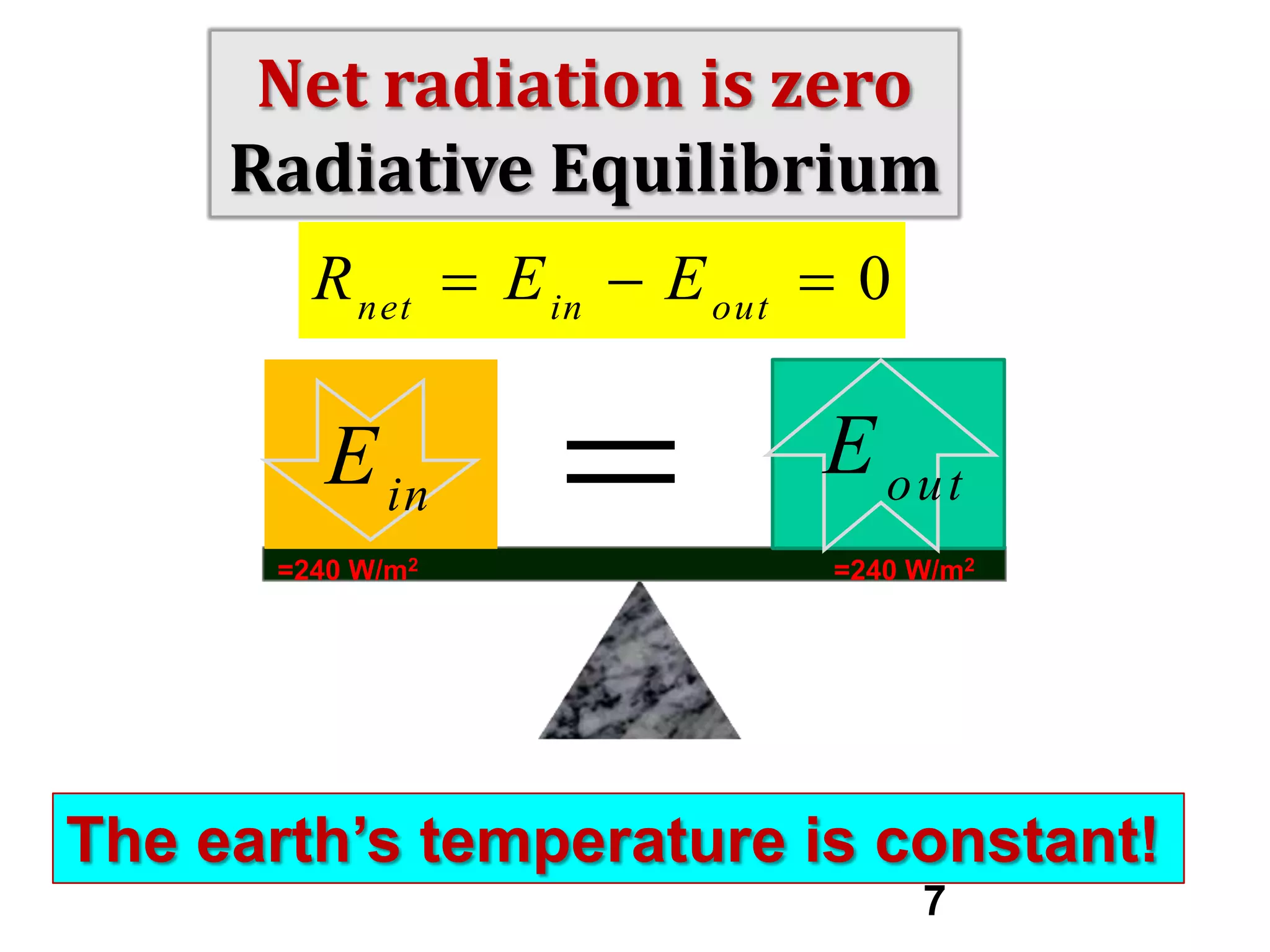
1) The document discusses the electromagnetic spectrum and the different types of radiation emitted by the sun and earth. It explains concepts like incoming shortwave radiation, outgoing longwave radiation, and how net radiation determines if the earth's temperature is increasing, decreasing, or in equilibrium.
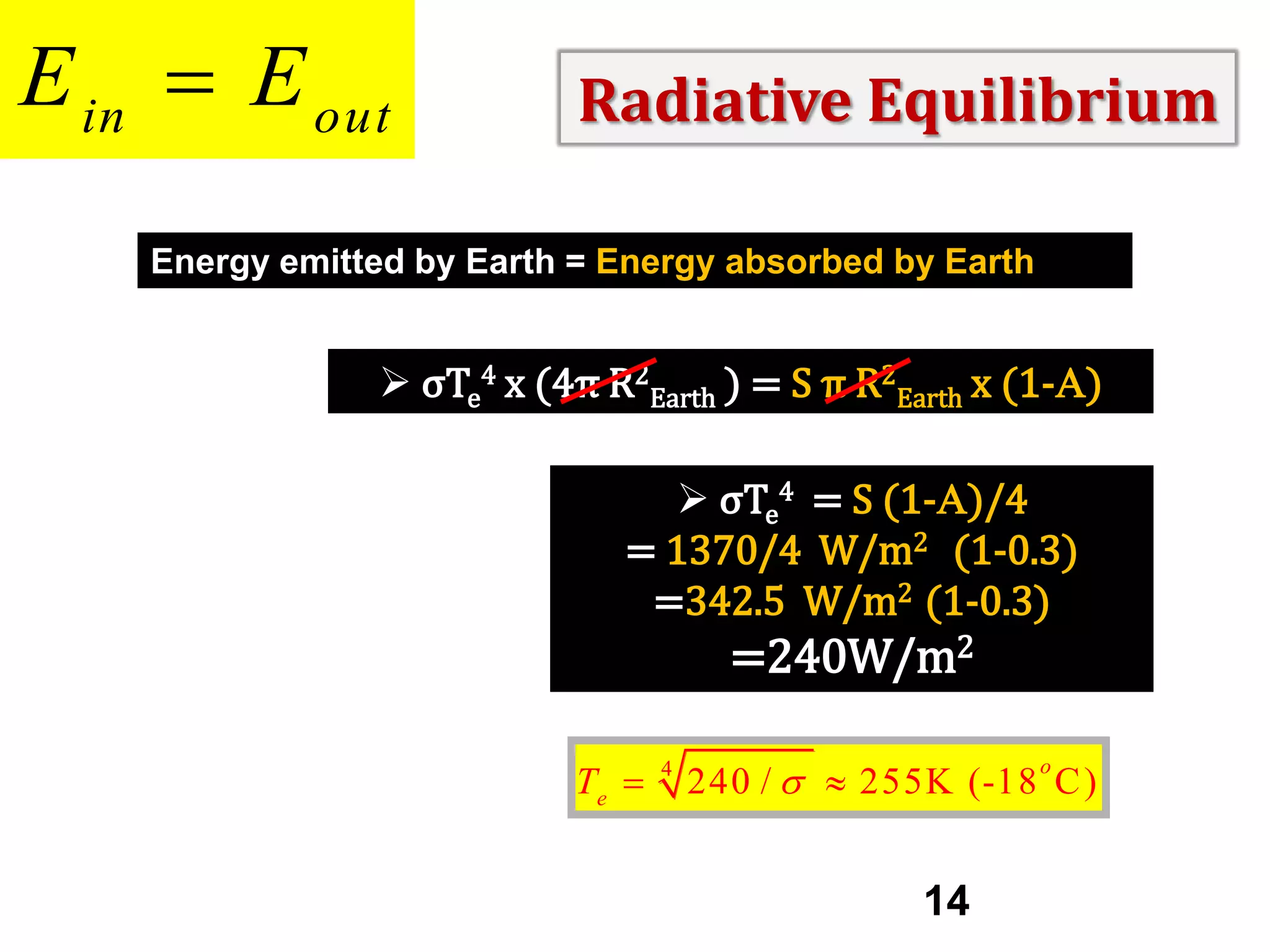
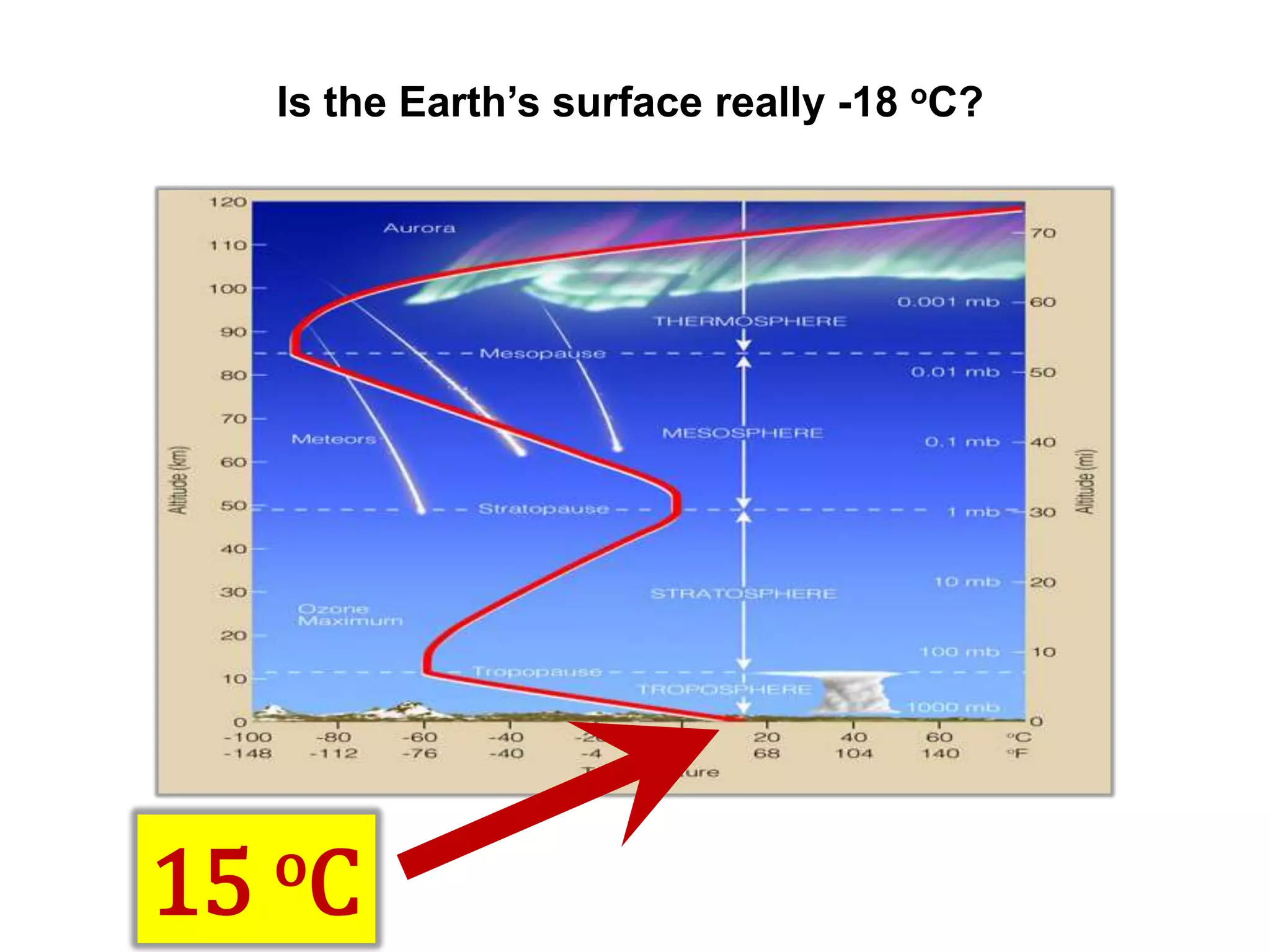
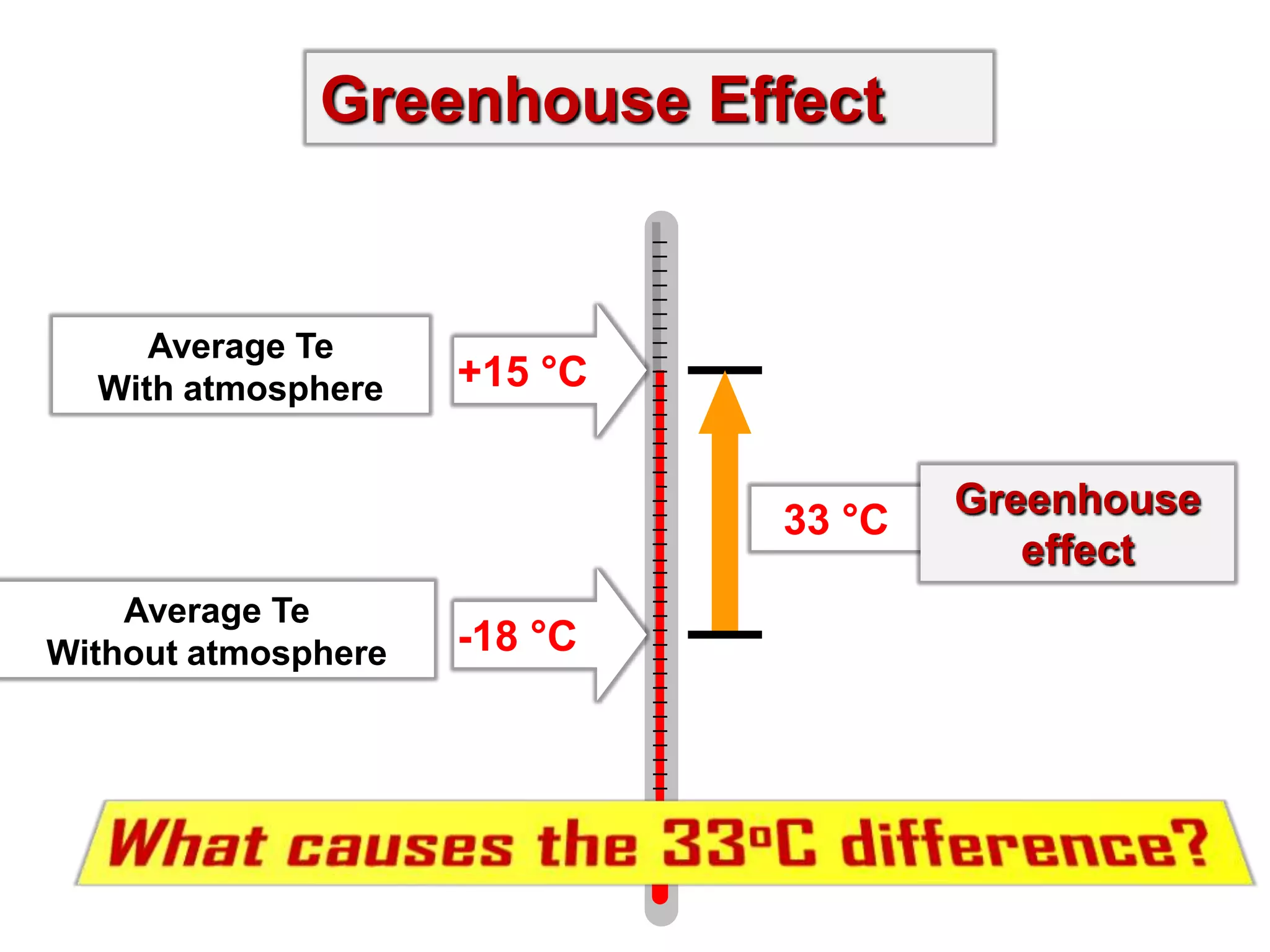
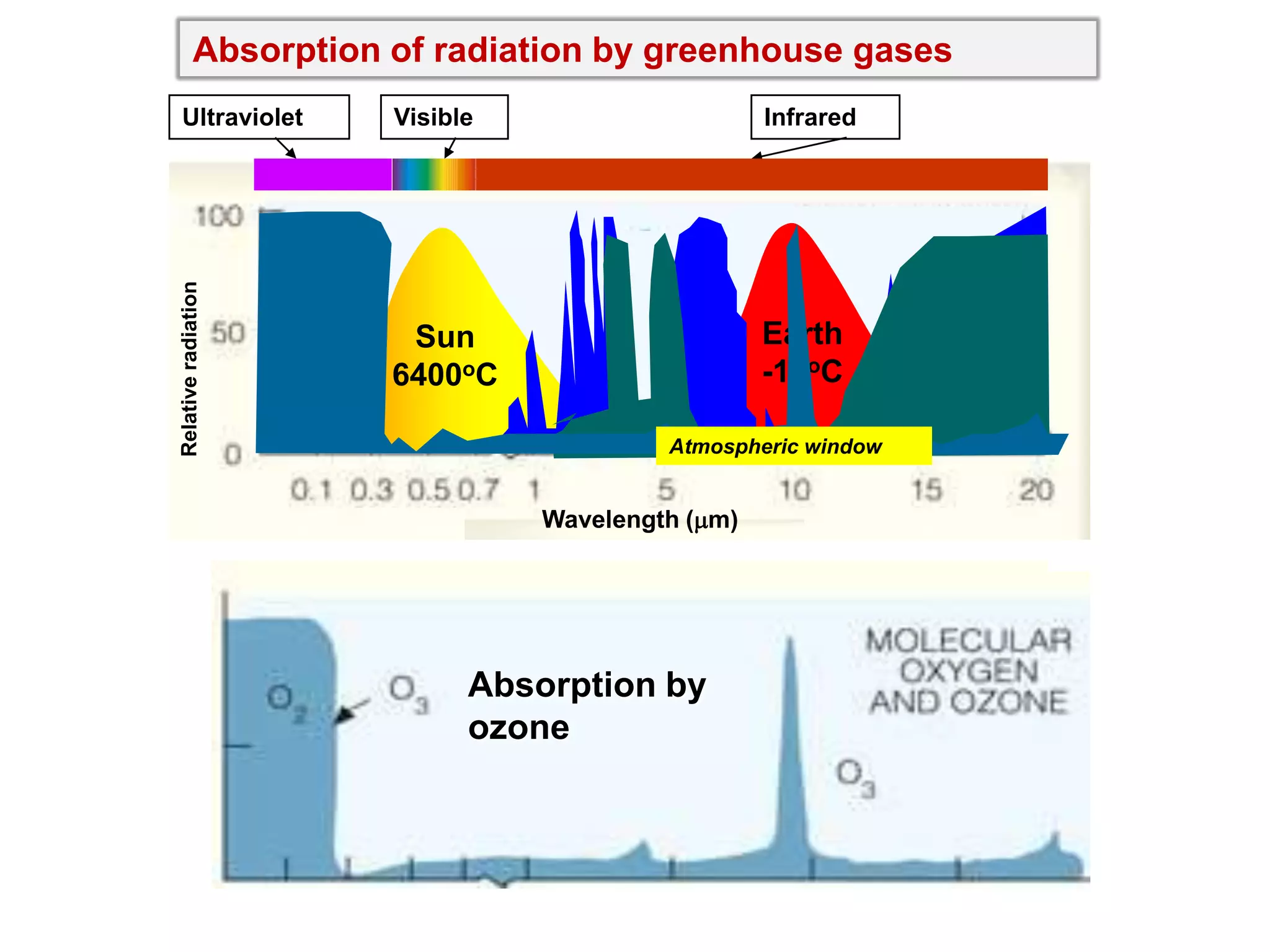
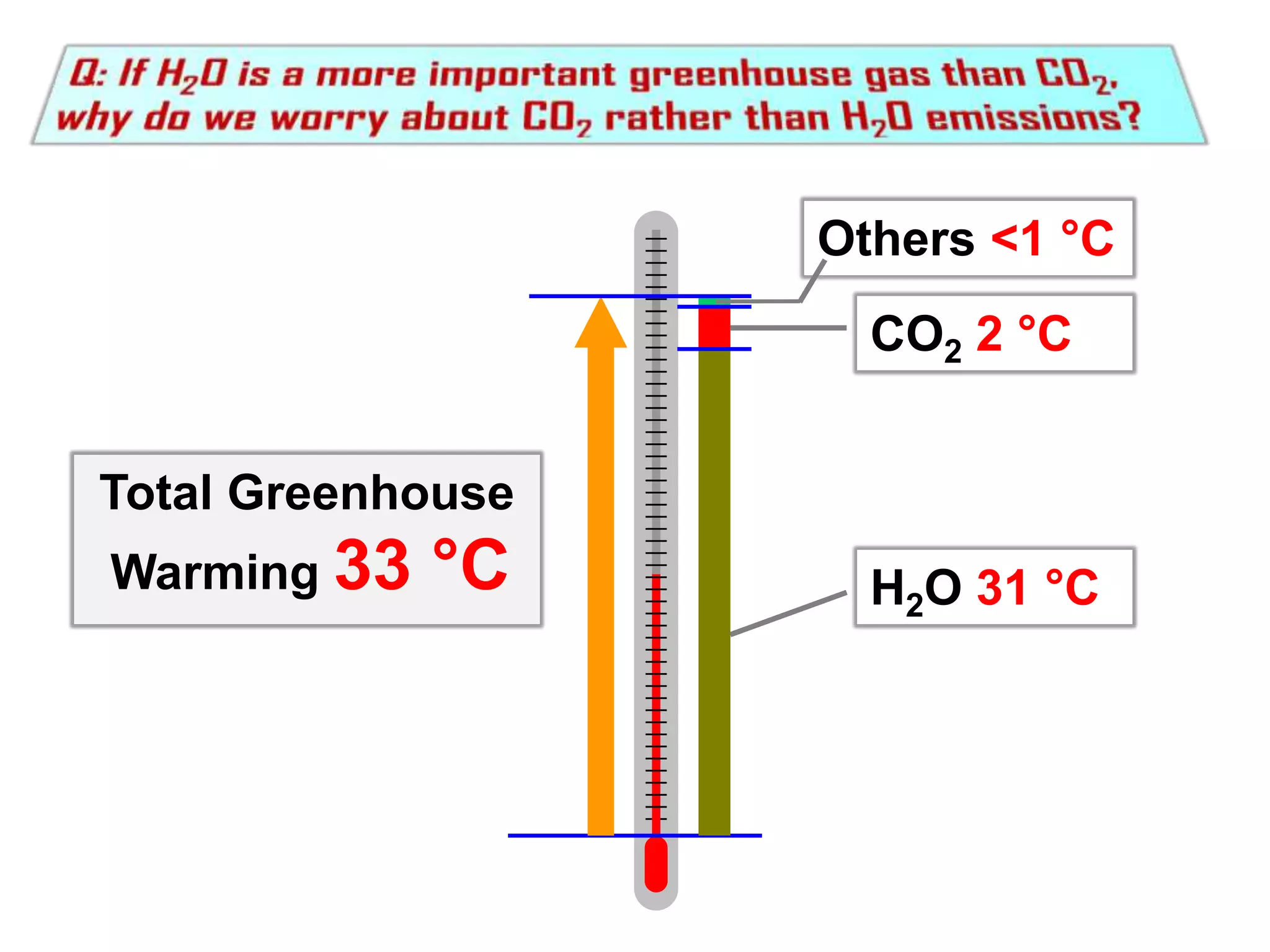
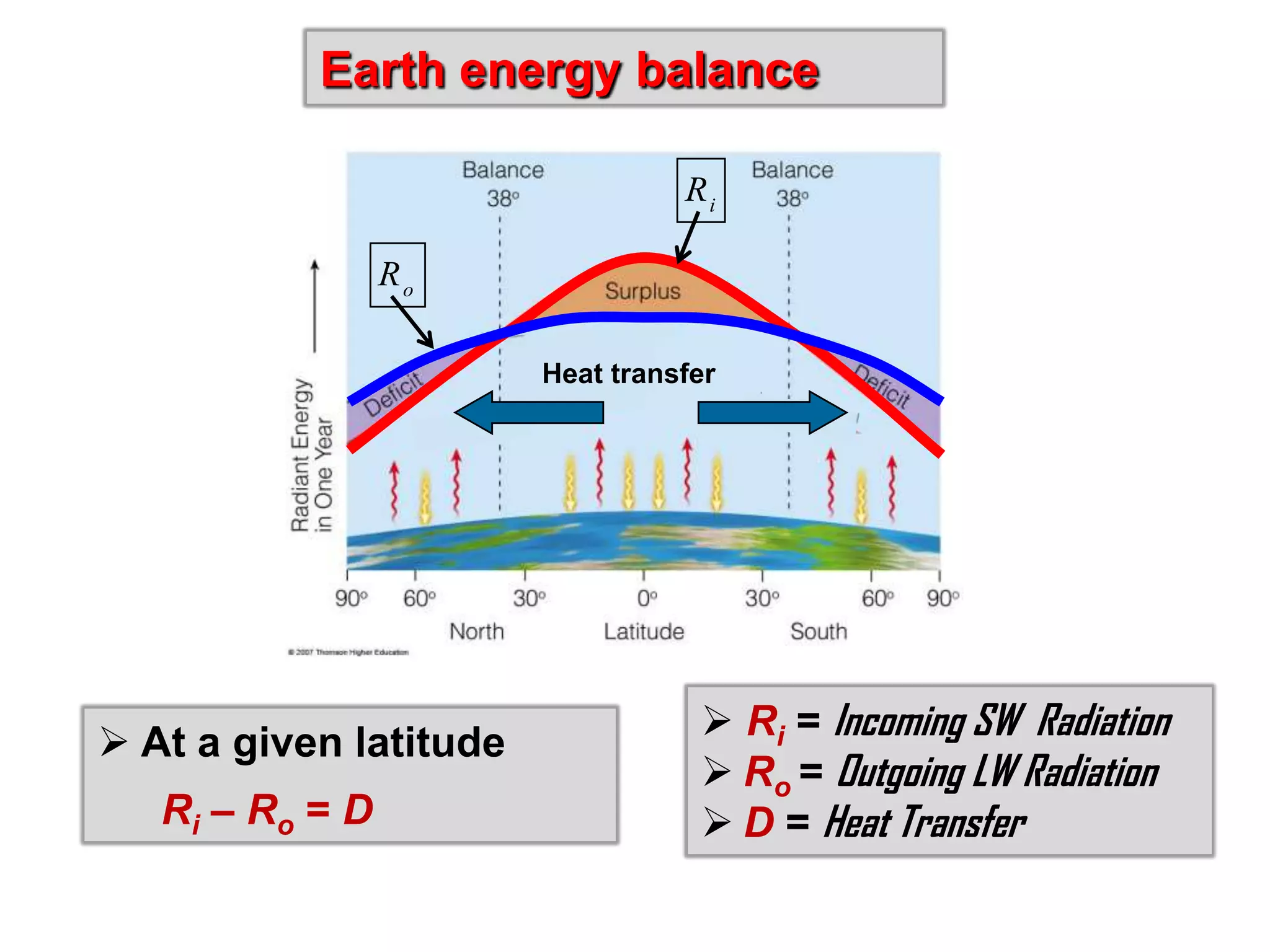
2) Key concepts covered include the greenhouse effect, how greenhouse gases like water vapor and carbon dioxide absorb and emit infrared radiation, and how this impacts the earth's energy budget and surface temperature.
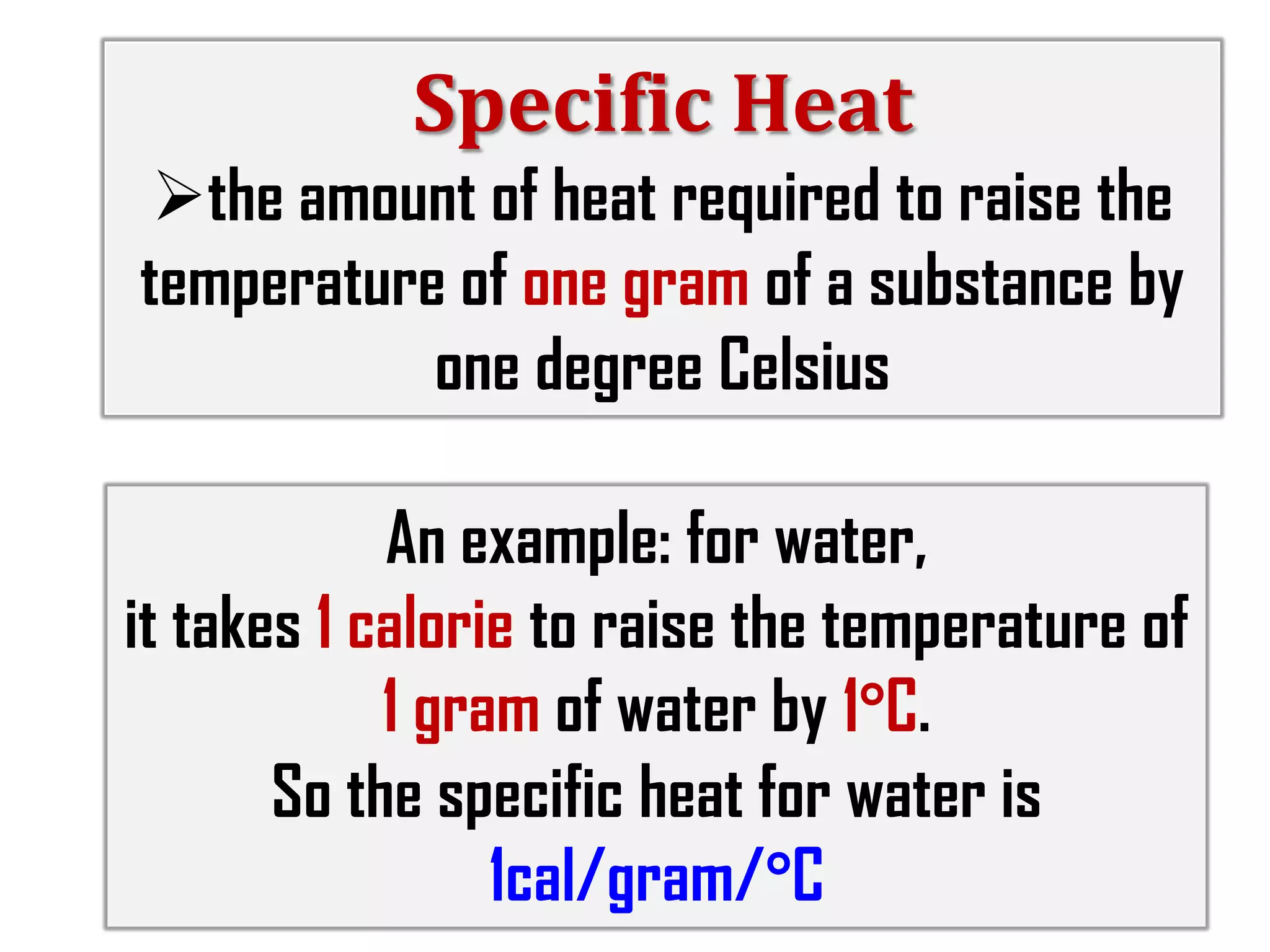
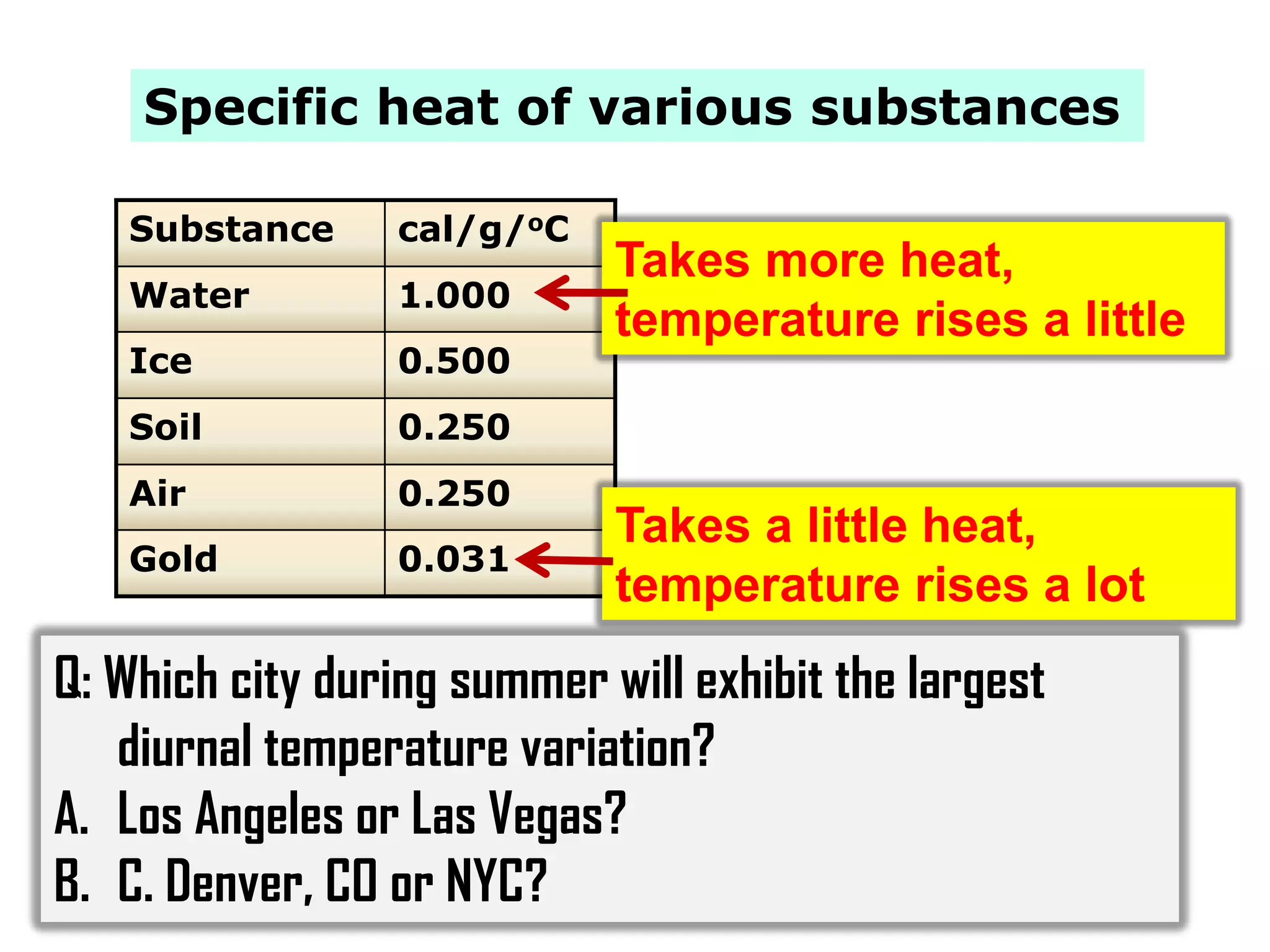
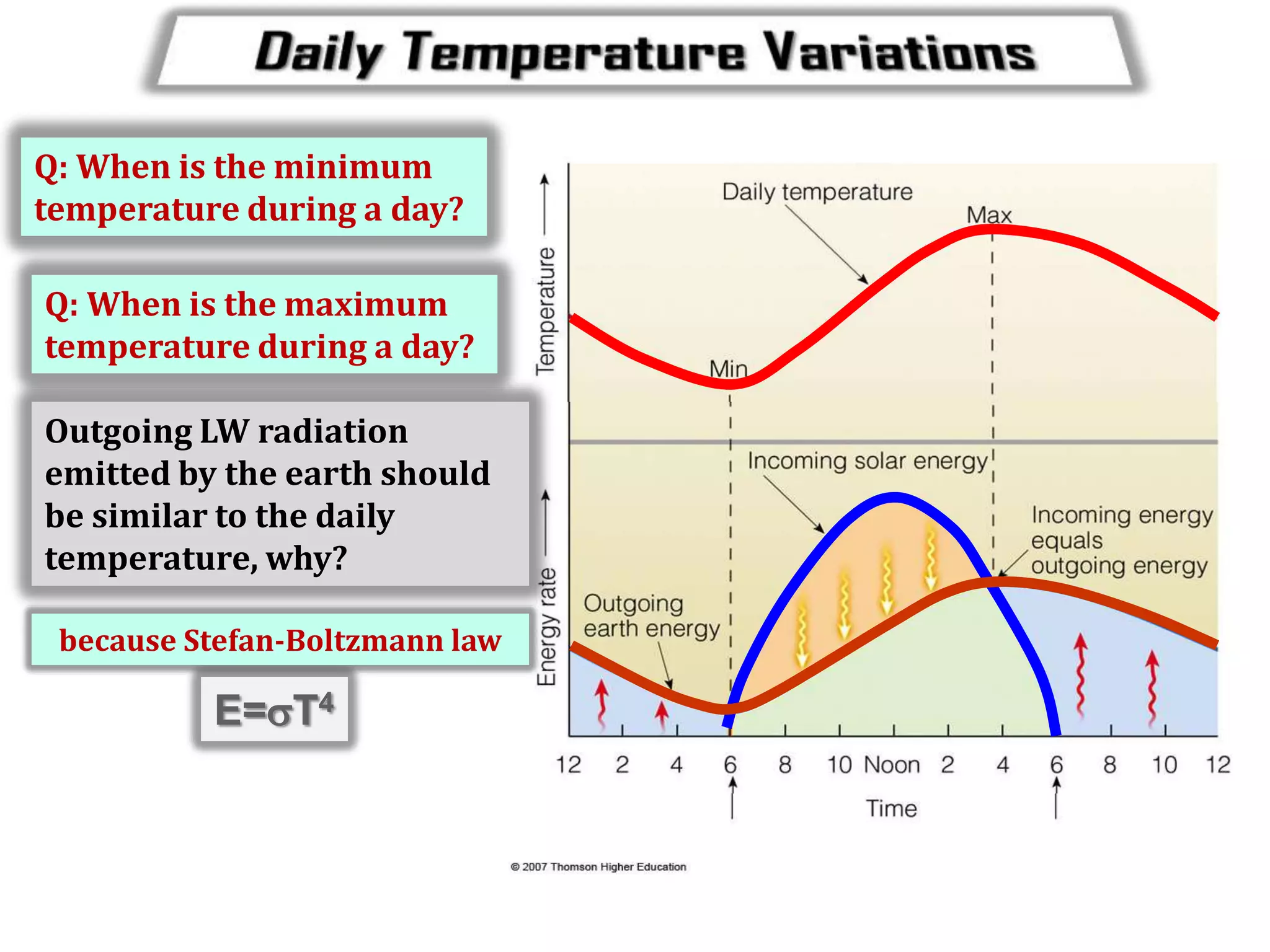
3) The document provides information on specific heat and how much energy is required to raise the temperature of different materials like water, ice, and air. It also discusses factors that influence diurnal temperature variations.
![E in
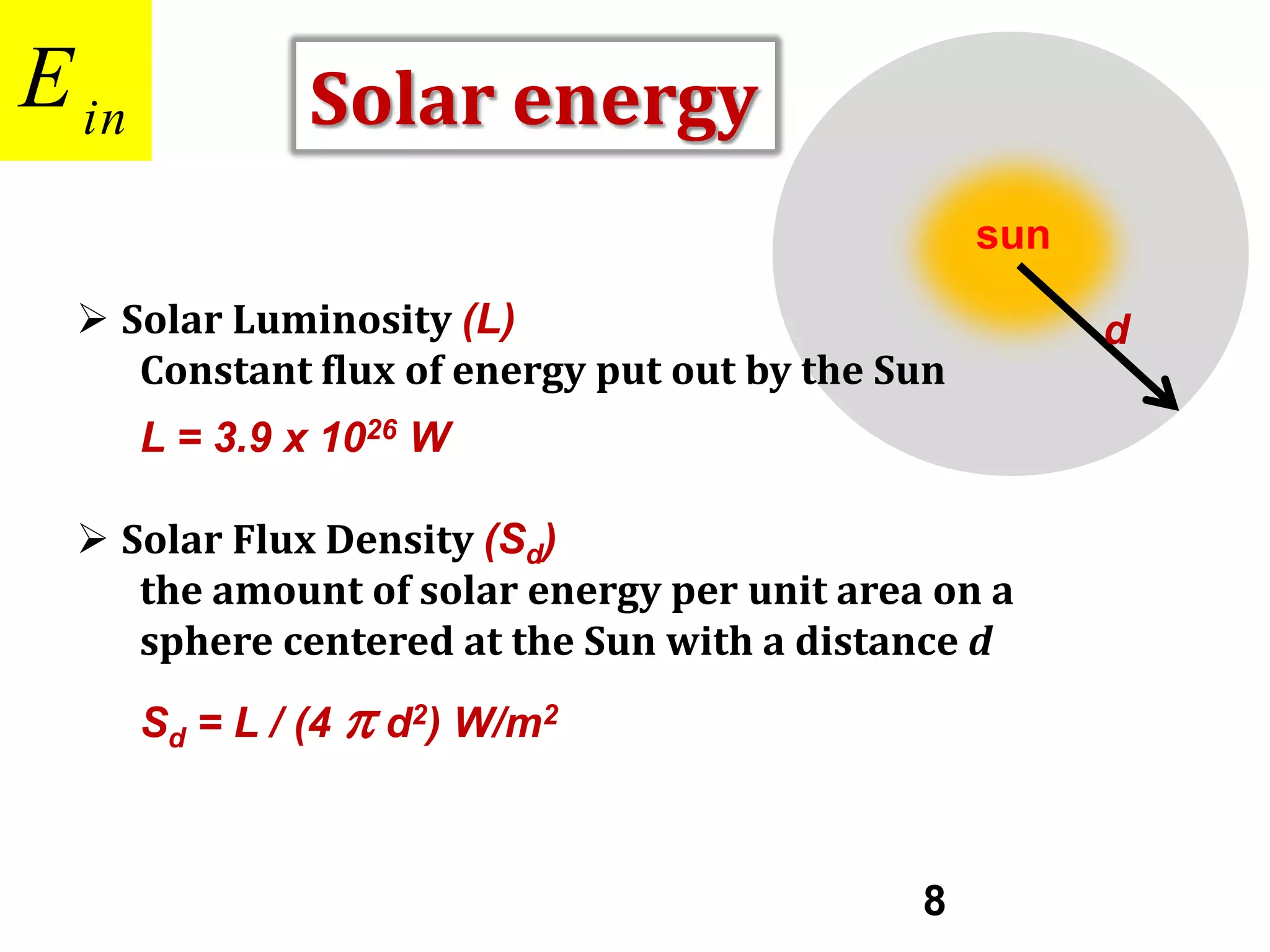
Incoming Short-Wave Radiation
Solar Constant (S )
The solar energy density at the
mean distance of Earth from the
sun (d =1.5 x 1011 m)
d
S = L / (4 π d2)
= (3.9 x 1026 W) / [4 x 3.14 x (1.5 x 1011 m)2]
= 1370 W/m2
Earth
9](https://image.slidesharecdn.com/lecture7-sep25-bb1-131217211038-phpapp01/75/Lecture7-sep25-bb-1-9-2048.jpg)