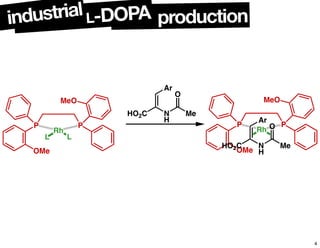
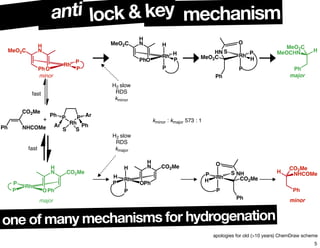
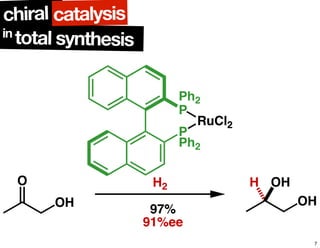
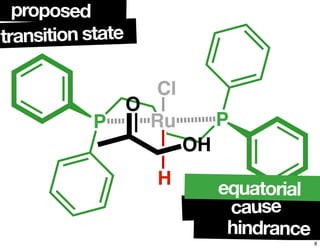
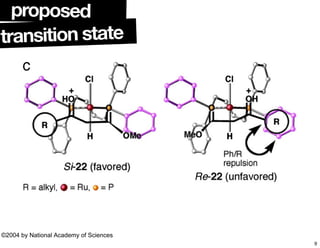
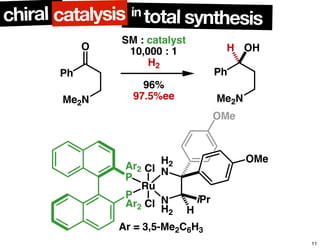
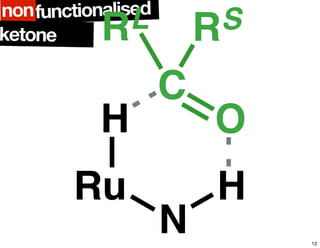
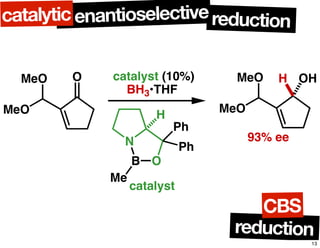
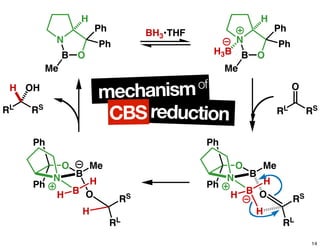
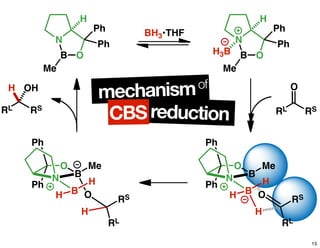
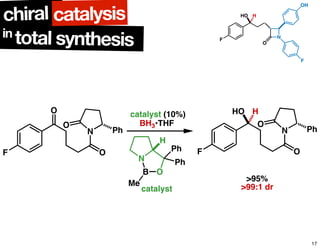
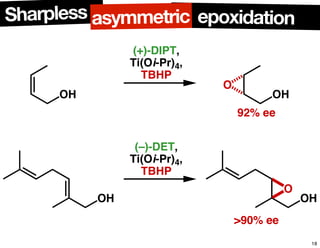
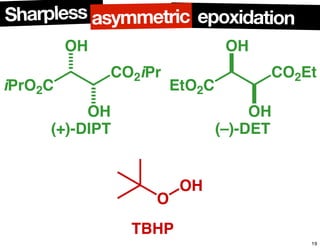
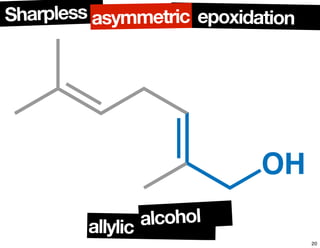
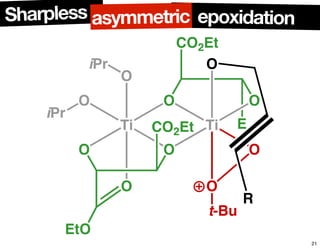
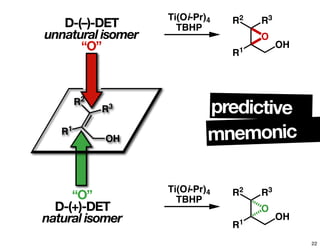
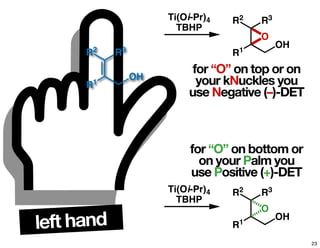
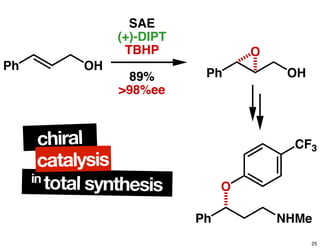
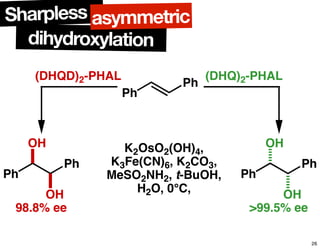
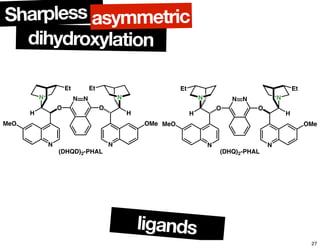
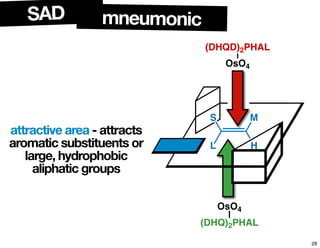
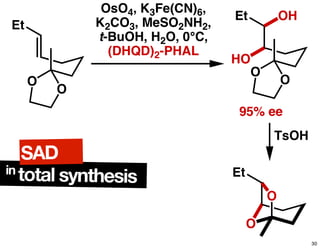
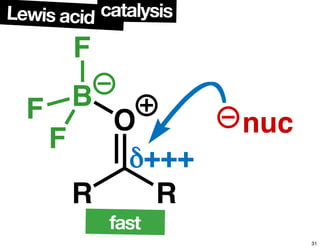
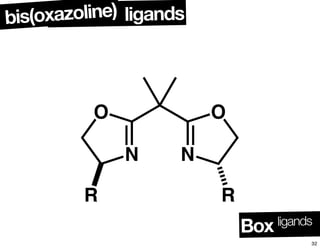
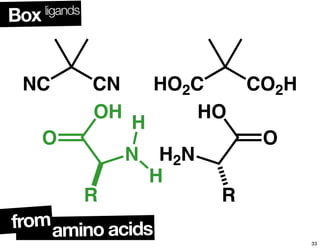
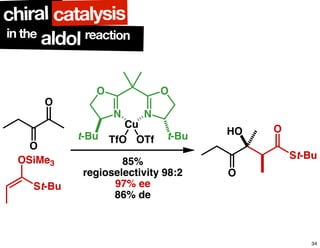
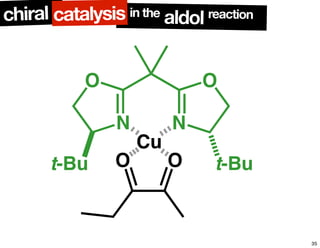
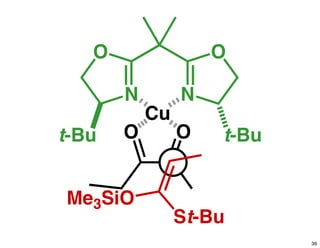
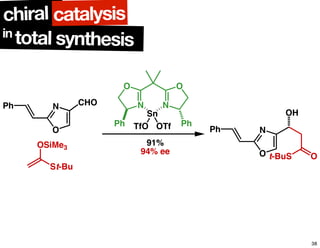
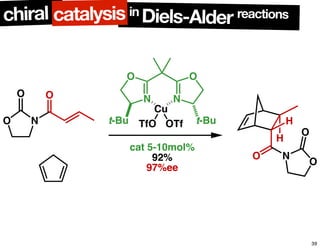
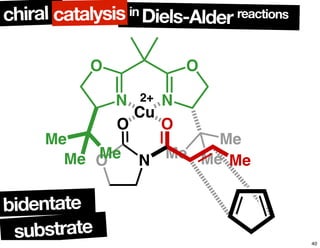
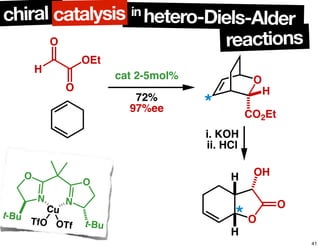
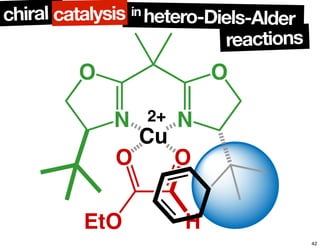
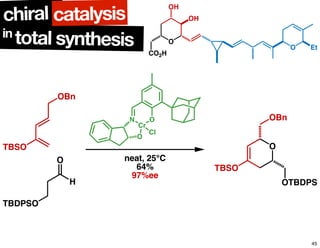
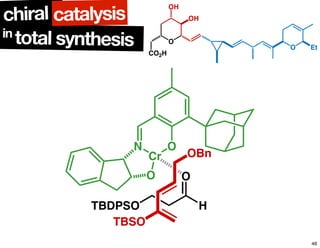
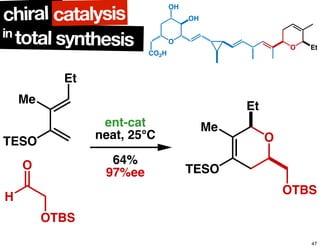
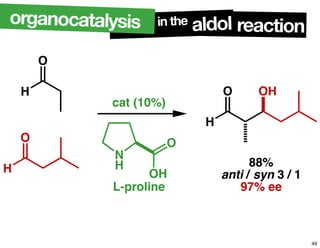
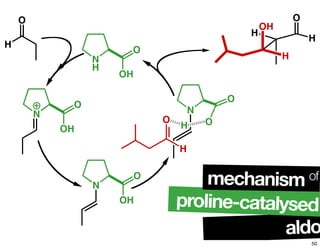
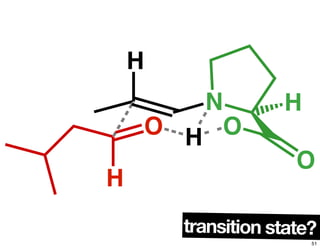
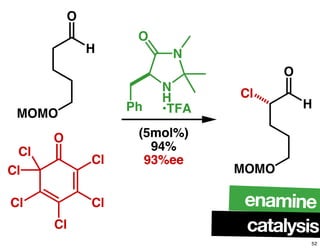
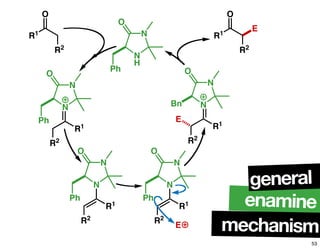
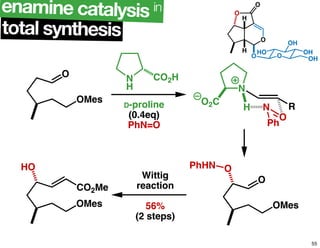
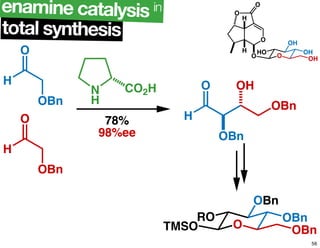
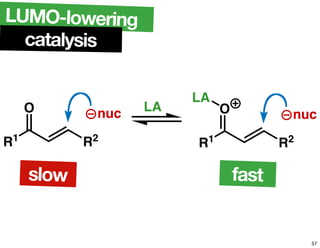
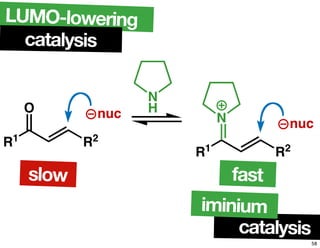
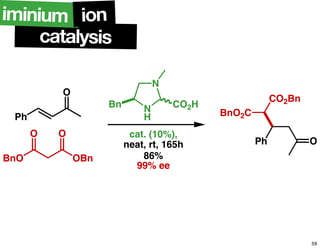
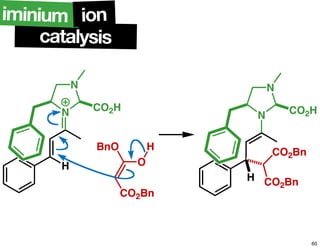
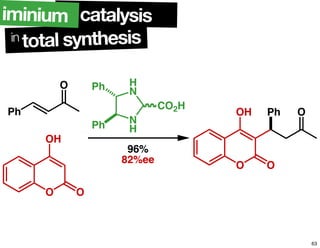
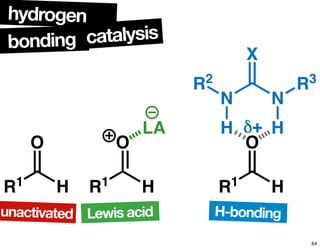
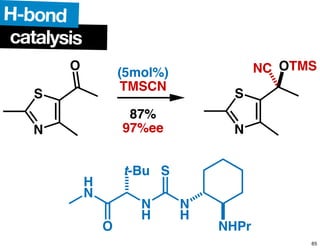
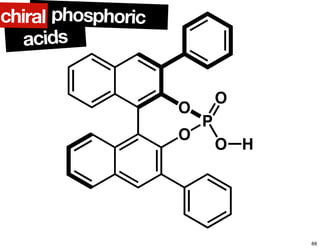
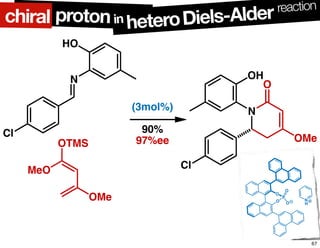
The document discusses chiral catalysis, detailing various reactions and mechanisms involved in the production of chiral compounds, such as L-DOPA and fluoxetine. It includes specific examples of reaction conditions, catalysts used, and efficiency metrics (e.g., enantiomeric excess). Additionally, it explores different methods and strategies for achieving selectivity in chiral synthesis.

![©mugley@flickr
H
CO2H
NHAc
MeO
AcO
H2(g)
[((S)-DIPAMP)RhL2]
L=solvent MeO
AcO
CO2H
H NHAc
H H
95% ee
(S,S)-DIPAMP
P P
OMe
MeO
industrial
production
L-DOPA
3](https://image.slidesharecdn.com/lecture2123713a-161117224330/85/Lecture2-123713A-3-320.jpg)