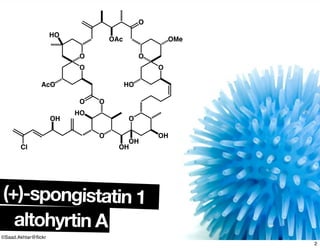
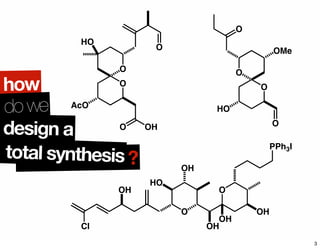
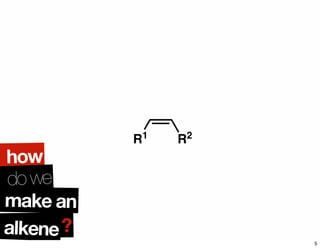
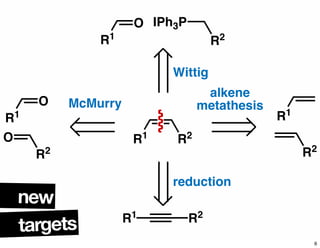
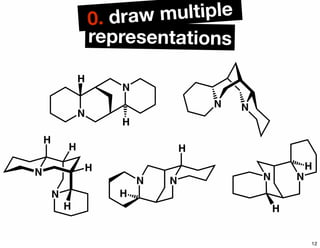
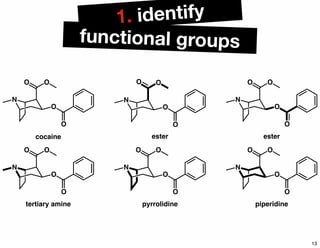
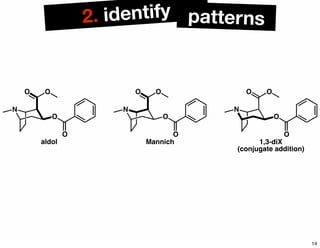
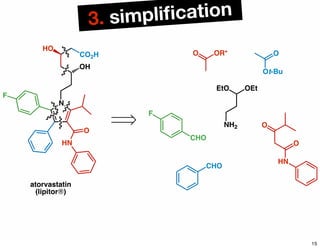
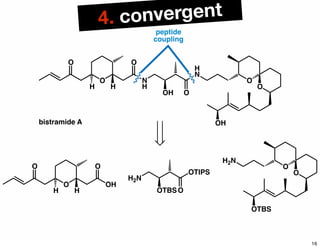
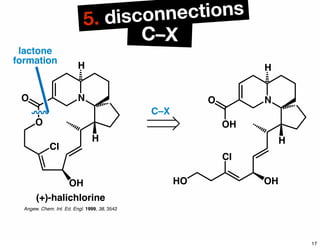
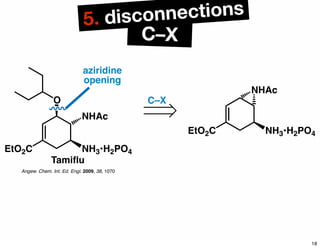
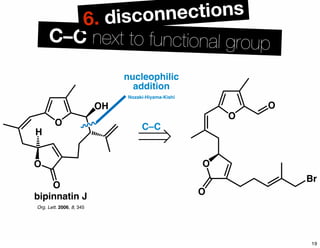
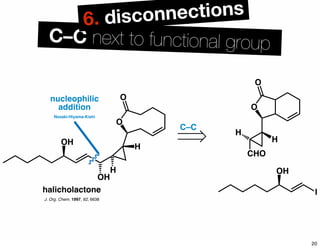
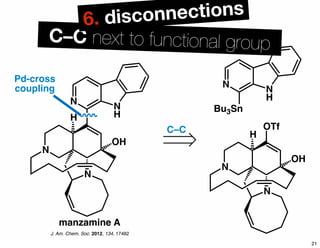
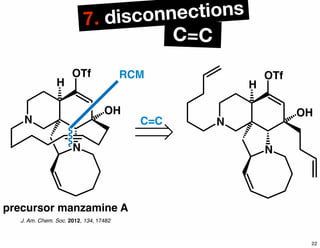
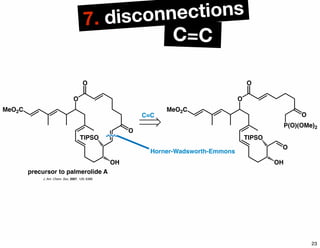
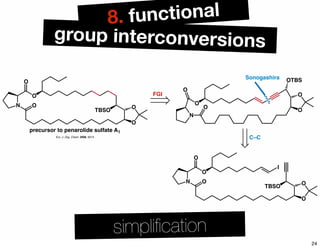
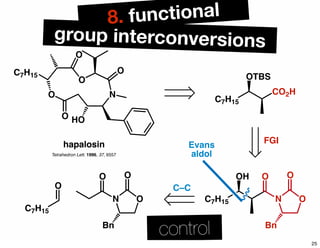
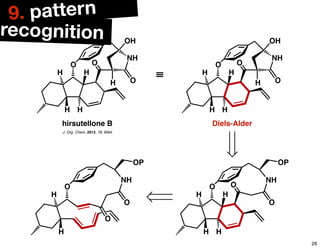
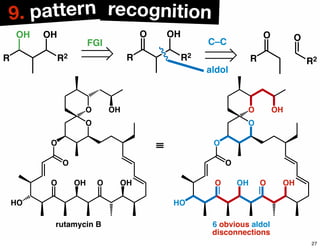
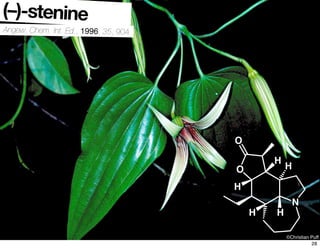
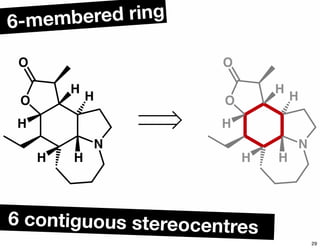
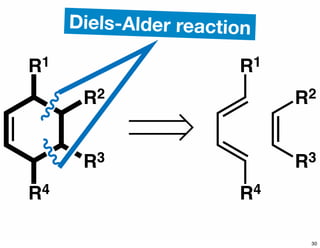
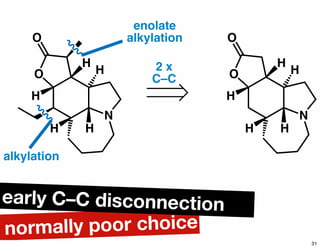
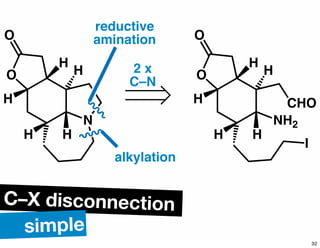
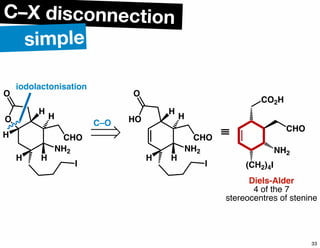
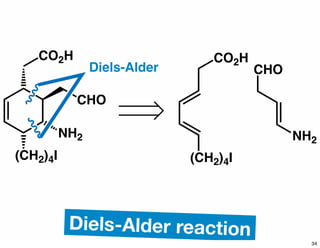
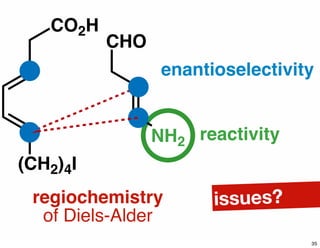
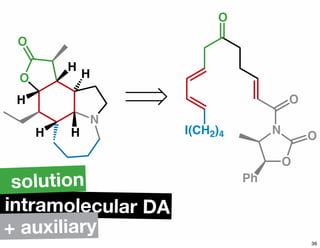
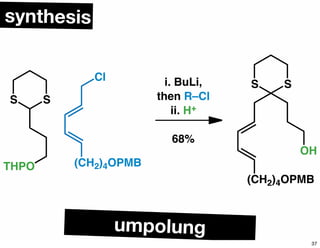
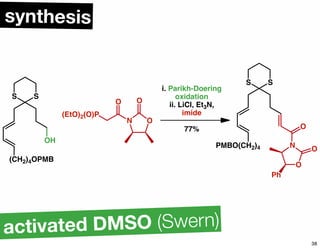
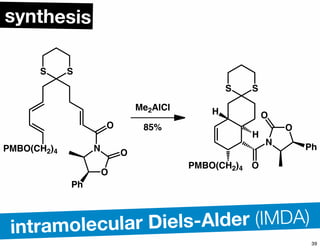
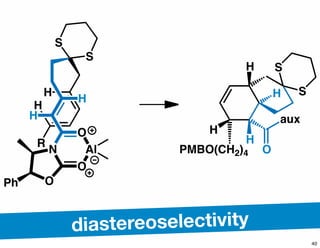
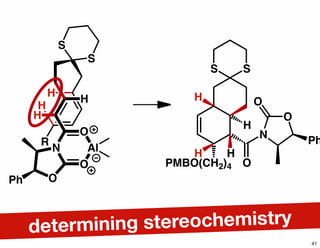
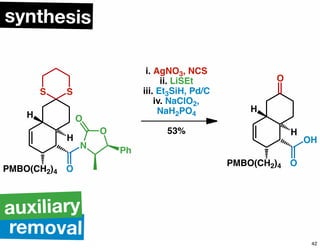
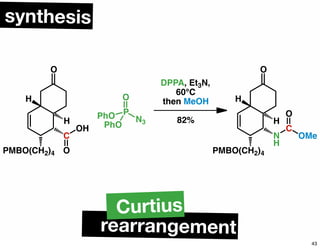
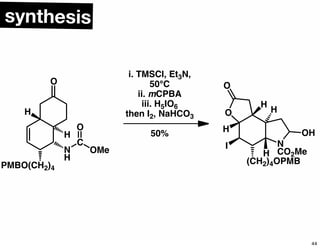
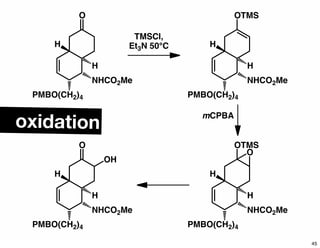
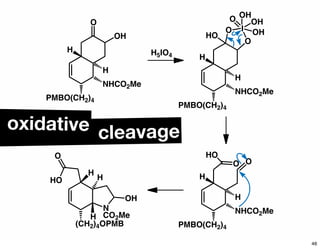
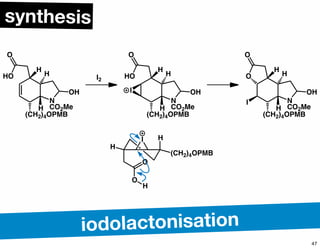
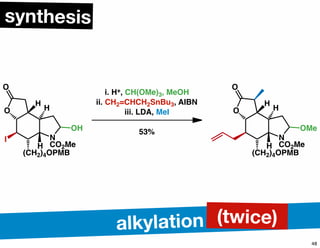
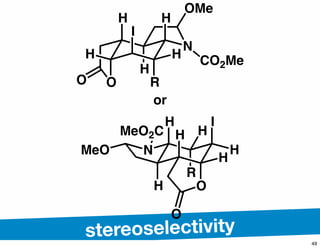
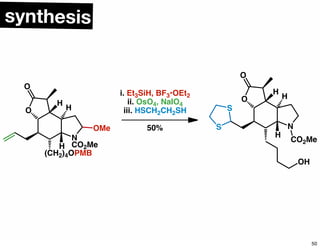
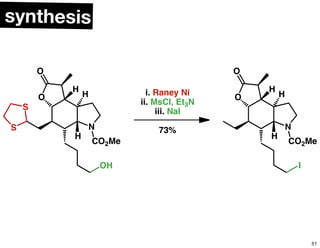
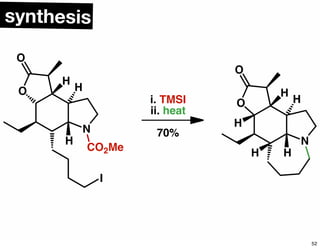
The document discusses various methodologies and strategies involved in total synthesis and retrosynthesis in organic chemistry, highlighting the importance of functional group identification, pattern recognition, and disconnection strategies. It provides specific examples and reaction schemes for various compounds, illustrating techniques such as aldol reactions, Diels-Alder reactions, and more. Additionally, the document emphasizes the need for simplification and convergent synthesis approaches in the design of synthetic pathways.