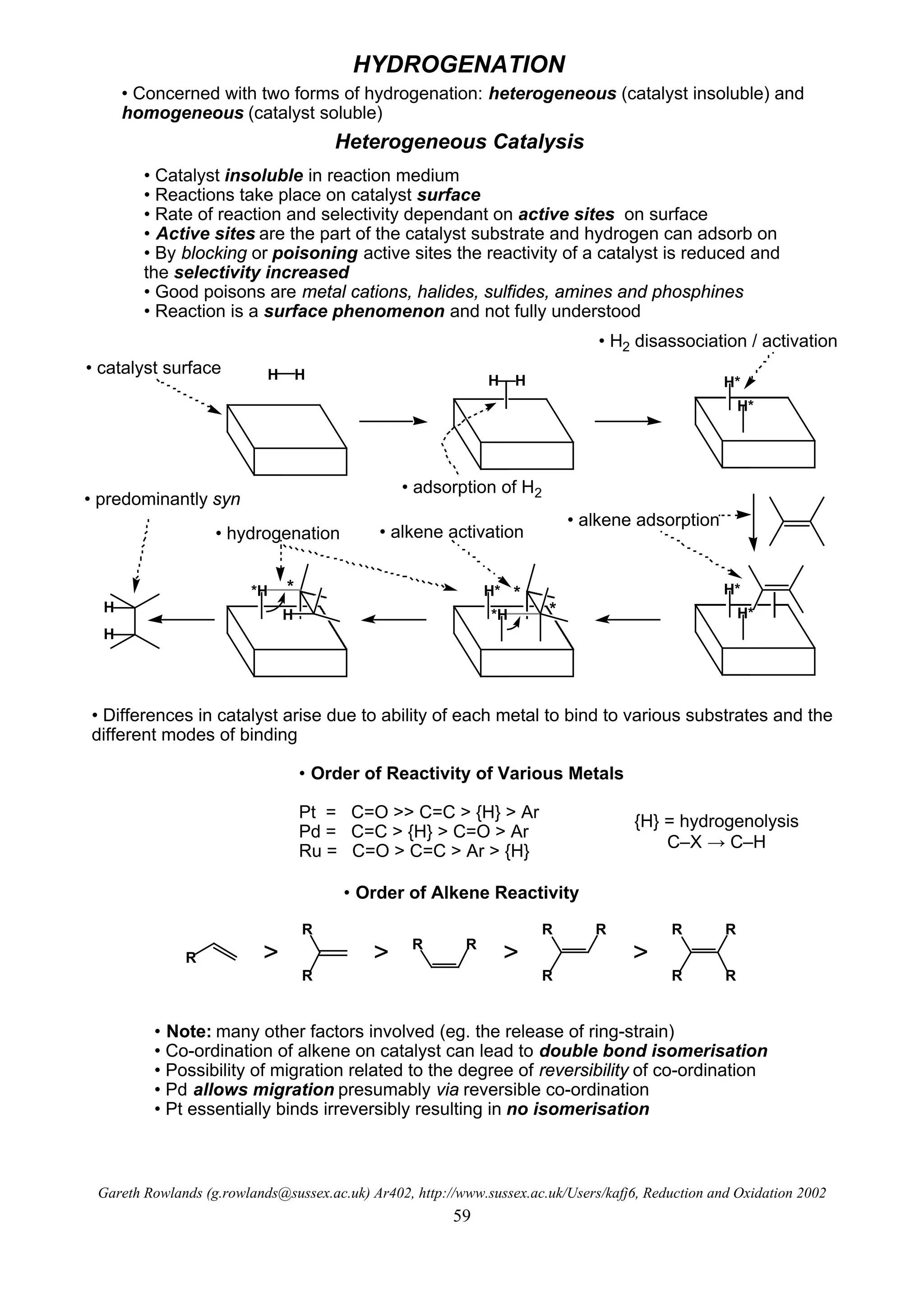
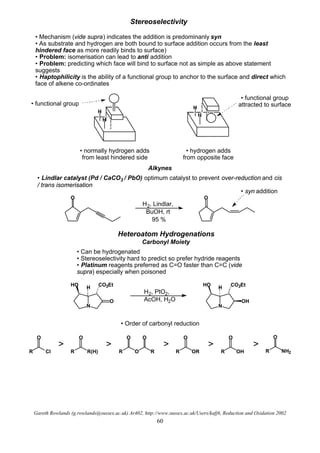
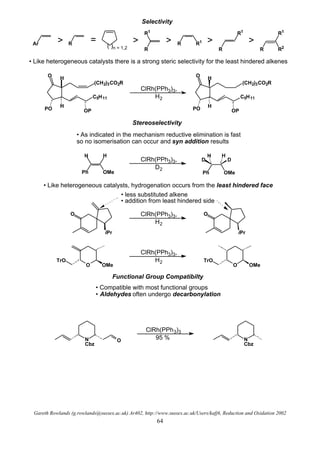
This document summarizes hydrogenation reactions using both heterogeneous and homogeneous catalysts. It discusses the mechanisms and selectivity of hydrogenation of alkenes, carbonyls, nitriles, nitro groups and azides using catalysts like platinum, palladium and rhodium. It also covers directed hydrogenation, asymmetric hydrogenation and transfer hydrogenation reactions. The order of reactivity for reducing different functional groups is provided.
![Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002
62
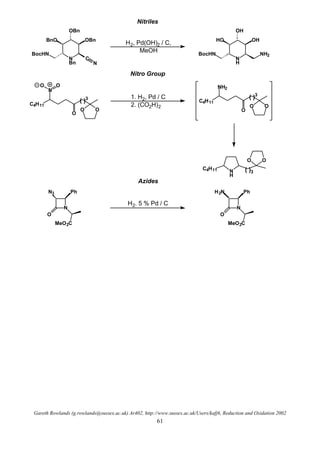
Homogeneous Catalyst
• Soluble in reaction medium
• Mechanisms much better understood
• Advantages: mild conditions (non-polar solvents which dissolve H2 better)
• Advantages: less catalyst required (each molecule is available for reaction and not just surface)
• Advantages: improved or complimentary selectivity (far more predictable)
• Advantages: directed hydrogenations
• Advantages: asymmetric hydrogenations
Alkene Hydrogenation
• 2 main types of homogeneous catalysts: dihydride and monohydride catalysts
Dihydride Catalysts
LnM + H2
H
LnM
H
• Examples: Wilkinson's Catalyst ClRh(PPh3)3 (hydrogen adds prior to substrate)
Crabtree's Catalyst [Ir(COD)(PCy3)(pyr)]+
PF6
–
(substrate adds before H2)
General Mechanism
LnM
H
MLnMLn
H
LnM H
LnM
H
LnM H
HH HH
H2
H2
reductive
elimination
reductive
elimination
• oxidative
cis addition
Wilkinson's
catalyst
Crabtree's
catalyst](https://image.slidesharecdn.com/hydr-200503073629/85/Hydrogenation-4-320.jpg)
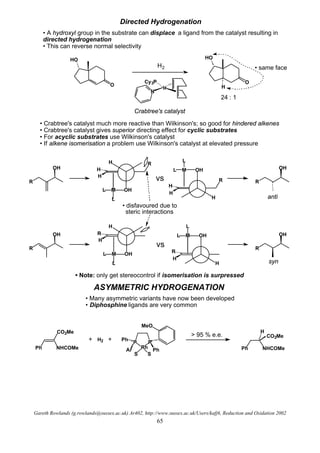
![Gareth Rowlands (g.rowlands@sussex.ac.uk) Ar402, http://www.sussex.ac.uk/Users/kafj6, Reduction and Oxidation 2002
68
R
Transfer Hydrogenation
O
OH
+
OH
O
+
N
ClN
HPh
Ph
Ts
Ru
• free NH crucial
• Mechanism is given in the Oxidation Section of this course
• Problem: the reaction is reversible (hence the oxidation)
• If formic acid / triethyl amine is used as the reductant reaction irreversible
N
H
N
H O
O
H+
N
H
NH +
O
C
O
cat.
Et3N
• gives off CO2
hence irreversible
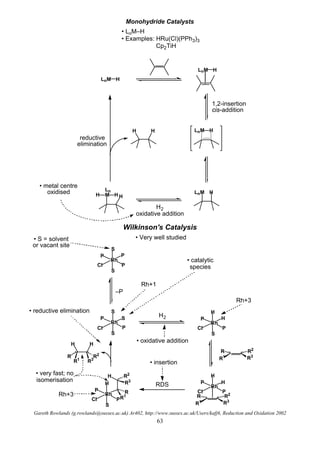
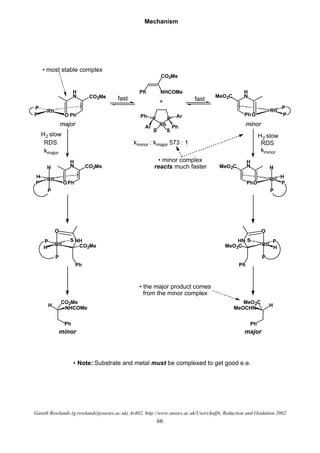
Hydrogenolysis
R X R H
H2
O
I
OMe
H
I
H2, Ni[R] O OMe
H
• Used to remove various functional groups
• Or protecting groups
O O R
OPh O
H2, Pd / C
O O R
OH O
Easiest
Hardest
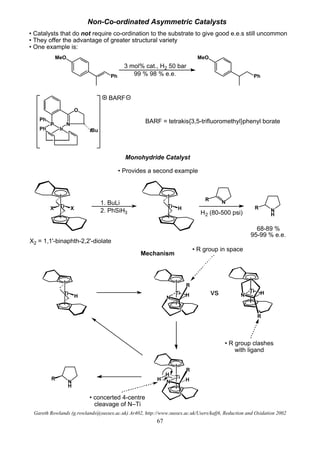
RCOCl RCHO
RNO2 RNH2
RC≡CR' RCH=CHR'
RCHO RCH2OH
RCH=CHR' RCH2CH2R'
RCOR' RCHOHR'
ArCH2OR ArCH3 + ROH
RC≡N RCH2NH2
RCO2R' RCH2OH + R'OH
Ease of reduction of functional groups towards catalytic hydrogenation
• note how far
down benzyl
group is
• Note: different catalysts have different propensities
for functional groups so this is only a rough order](https://image.slidesharecdn.com/hydr-200503073629/85/Hydrogenation-10-320.jpg)