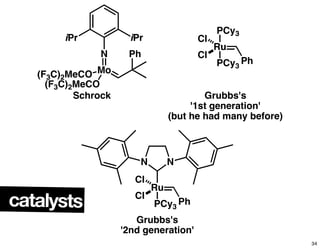
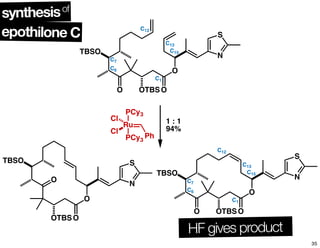
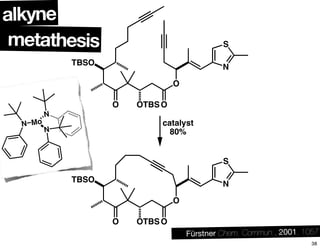
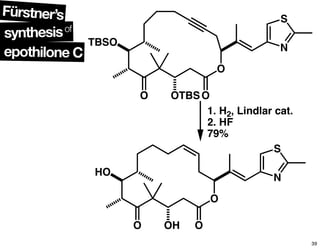
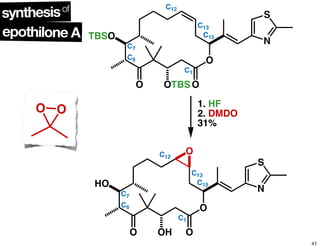
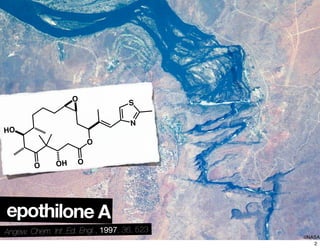
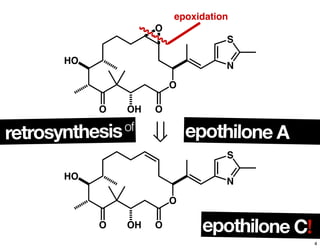
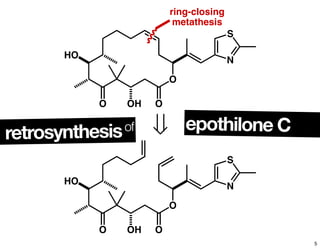
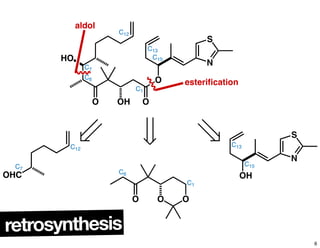
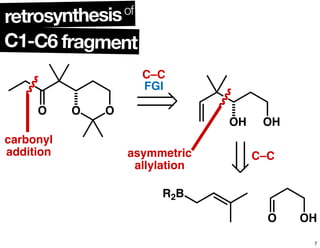
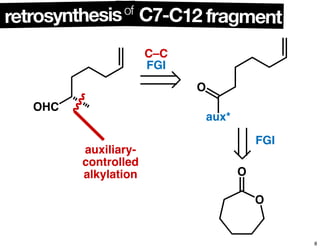
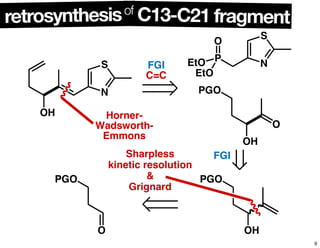
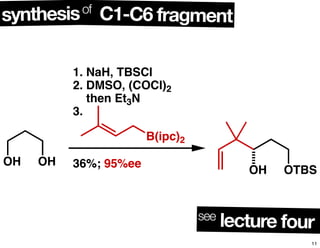
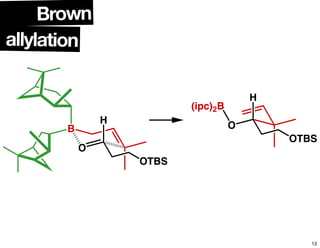
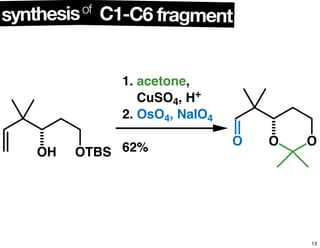
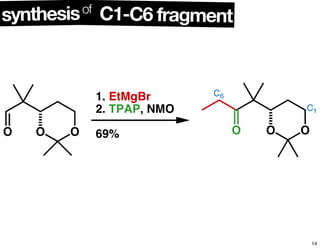
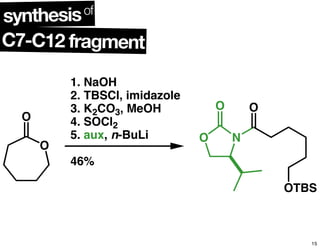
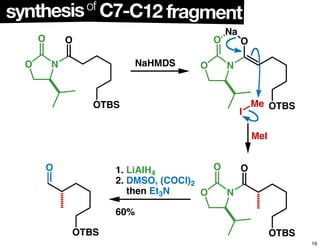
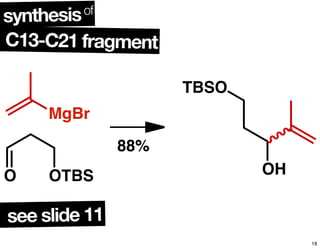
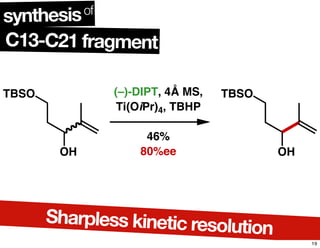
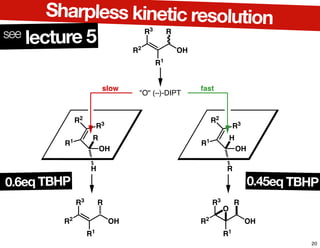
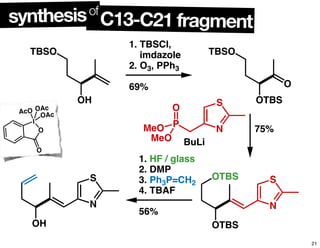
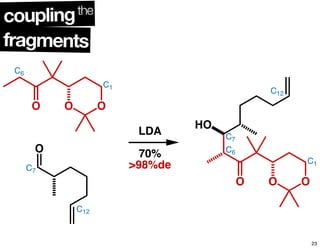
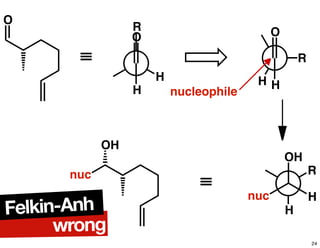
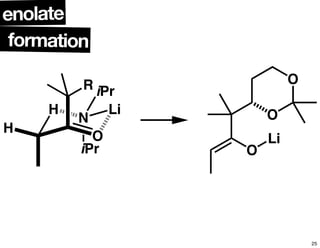
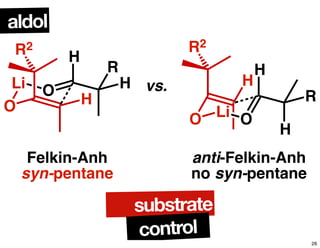
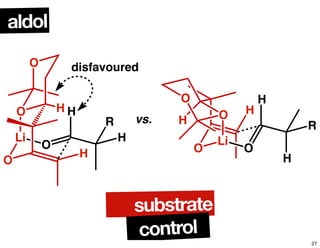
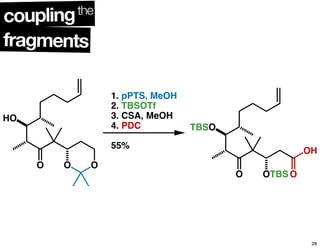
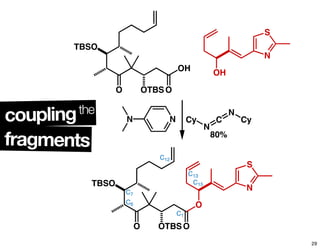
This document summarizes the synthesis of the anti-cancer compound epothilone A. It discusses the retrosynthesis of epothilone C and the synthesis of the required fragments - C1-C6, C7-C12, and C13-C21. These fragments were coupled and the ring was formed using ring-closing metathesis. Finally, epothilone C was converted to the target compound epothilone A through oxidation and reduction reactions. The synthesis utilized substrate-controlled aldol reactions, Sharpless asymmetric dihydroxylation, and ring-closing metathesis to construct the molecule with high stereoselectivity.




![R2 R1
[M]
R1
R2
[M]
[M]
R1
[M]
R1
[M]
R1
R2
R2
H
H H
H
32](https://image.slidesharecdn.com/lecture4123713b-161117225410/85/Lecture4-123713B-32-320.jpg)
![[M]
[M]
[M]
H
H H
H
[M]
[M]
H
H H
H
ring-closing
metathesis
RCM
33](https://image.slidesharecdn.com/lecture4123713b-161117225410/85/Lecture4-123713B-33-320.jpg)