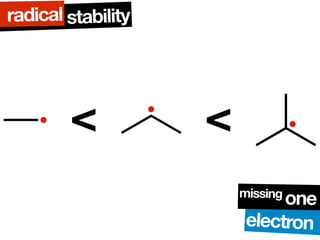
The lecture discusses the mechanisms of ozonolysis and radical addition reactions to alkenes. Ozonolysis involves a three step mechanism where ozone cleaves the alkene to form an ozonide intermediate which then decomposes to a carbonyl compound. Radical addition reactions involve a three step chain reaction mechanism of initiation, propagation, and termination. The stability of radical intermediates is influenced by resonance stabilization, which explains why styrene reacts with HBr to give a single, benzylic bromide product.