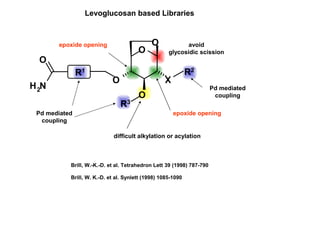
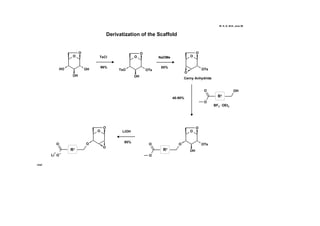
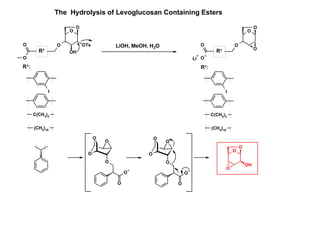
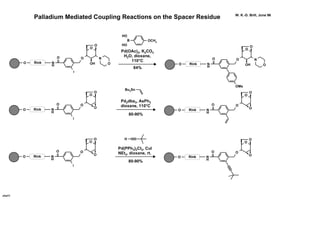
This document describes the catalysis of substitution reactions on heterocycles bound to a solid phase. It discusses immobilizing 2,6-dichloropurine on a Rink resin, performing C6-substitution with various amines in high yields, and C2-substitution via palladium-mediated coupling reactions such as Suzuki and amination reactions. It also investigates modifying the polymeric support by treating it with a brominating agent to install bromine complexes on the resin for further substitution chemistries. The reactivity of the bromine complex is explored, finding it performs selective side reactions in dry benzene that are not possible in NMP solvent.












![Yield[%]Entry: Solvent Catalyst T [°C] Reaction
Time [h] Start.mat. Product
1 dioxane DIPEA 80 24 100 -
2 2,6-lutidine/dioxane - 60 24 79.3 24.3
3 2,6-lutidine/NMP - 60 24 31.7 68.3
4 “ HNEt3
+
F3CCO2
-
60 24 11.7 88.3
N
H
F
Cl
N
N
N
H
N
Cl
N
F
Cl
N
H N
N
N
Cl
Cl
C6-Substitution](https://image.slidesharecdn.com/cae95f04-0fc0-4f0a-beab-78f99ab19d6c-160926113620/85/catalysis_of_substitution_reactions_on_heterocycles_on_sp-17-320.jpg)

![N
N N
N
Cl
N
N
Cl
N N
N
N
N
O
N
N
Cl
N
H
O
Pd-catalyst
N
N N
N
Cl
N
N
N N
N
N
N
N
B
OH
OH
Pd-catalyst
Solvent Base
Cat. or
Promotor
Co-ligand
Reaction
T [°C]
Time
[h]
Yield
[%]
NMP DIPEA - - 100 48 -
NMP Cs2CO3 Pd(PCy3)2Cl2 - 100 “ 48.7
“ K3PO4 “ - “ “ 45.7
“ Cs2CO3 Pd2dba3 P(tBu)3 “ “ 65.6
“ K3PO4 “ “ “ “ 66.3*
Solvent Base
Cat. or
Promotor
Co-ligand
Reaction
T [°C]
Time
[h]
Yield
[%]
NMP K3PO4 Pd2dba3 P(tBu)3 100 48 84.5*
Sustitution on C2
* rest is starting mat.](https://image.slidesharecdn.com/cae95f04-0fc0-4f0a-beab-78f99ab19d6c-160926113620/85/catalysis_of_substitution_reactions_on_heterocycles_on_sp-19-320.jpg)


![Entry Resin Conditions Time [h] Bromine content
1 Merrifield Br-complex, NMP, 2,6 lutidine 3x 24
1
<0.3 %
2 Merrifield Br-complex benzene,
2,6-lutidine
24 1.1% 0.14mmol/g
3 product of
entry 2
sat. KOtBu, dioxane 88°C 72 <0.3%
1
) Three consecutive treatments
.
Modification of the Polymeric Support by the Brominating agent
N
Br2
Bromine-complex
Br-content of resin, treated with the Bromine Complex (EA)
N
Br2
Br](https://image.slidesharecdn.com/cae95f04-0fc0-4f0a-beab-78f99ab19d6c-160926113620/85/catalysis_of_substitution_reactions_on_heterocycles_on_sp-22-320.jpg)





![Entry Solvent Cu cat. Pd cat. Co-ligand
Product
yield
[%]
dehalog.
[%]
1 dioxane - Pd2dba3 As(Ph)3 - >5
2 NMP CuO Pd2dba3 dpppf
no reaction
3 NMP Cu(OAc)2 Pd2dba3 dppp - 91.6
4 NMP
O
Bu
Et
O
2
Cu
2+
Pd2dba3 dppp 51.5 48.5
5 NMP CuI Pd2dba3 dppp 20 -
6 NMP S
CuO
O
Pd2dba3 dppp 35.7 64.1
7 NMP Cu2O - - no reaction
8 NMP Cu2O Pd(OAc)2 dppp >98 -
SnBu3
N
NN
N
Br
NH
NH N
NN
N
NH
NH N
N
N
N
NH
NH
cat.
+
Substitution on C8
P P
dppp:](https://image.slidesharecdn.com/cae95f04-0fc0-4f0a-beab-78f99ab19d6c-160926113620/85/catalysis_of_substitution_reactions_on_heterocycles_on_sp-28-320.jpg)



















