This document discusses the structural aspects of monosaccharides, including:
- Monosaccharides can exist as stereoisomers that have the same structural formula but different spatial configurations. The number of asymmetric carbon atoms determines the possible isomers.
- Glyceraldehyde is the simplest monosaccharide and is used as a reference to represent carbohydrate structures. It exists as two stereoisomers.
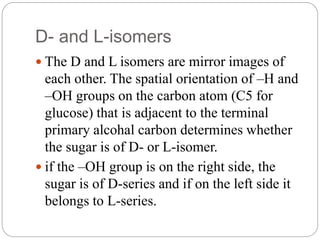
- Monosaccharides can be either D- or L-isomers depending on the spatial orientation of groups on an adjacent carbon. Naturally occurring monosaccharides are mostly the D-configuration.
- Optical activity is a characteristic of compounds with asymmetric carbon atoms. D- and L- configurations relate to