1. This document provides an overview of biochemistry for graduate study, covering the four main types of biomolecules - carbohydrates, lipids, nucleic acids, and proteins.
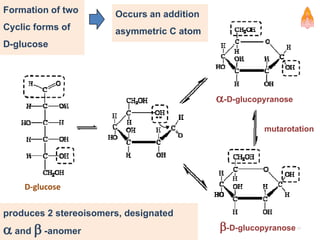
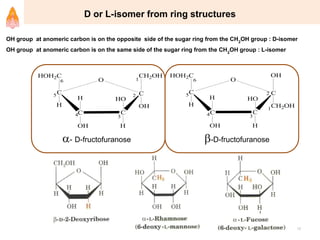
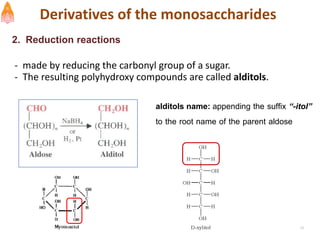
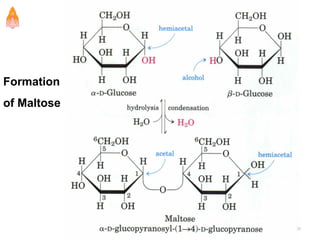
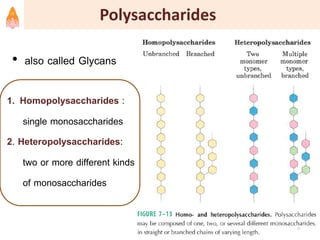
2. It describes carbohydrates in detail, explaining their monomer units, classifications, structures, properties and biological roles. This includes monosaccharides, oligosaccharides, polysaccharides, and glycoconjugates.
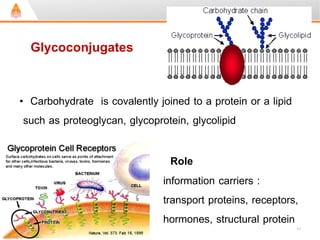
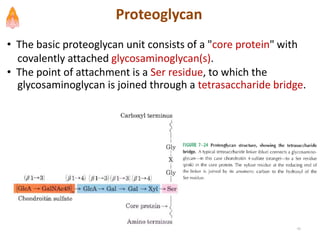
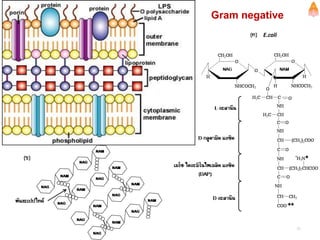
3. Key polysaccharides discussed are starch, glycogen, cellulose, chitin, glycosaminoglycans, and peptidoglycan. Glycoconjugates such as proteoglycans, glycolipids, and glycoproteins are also summarized.