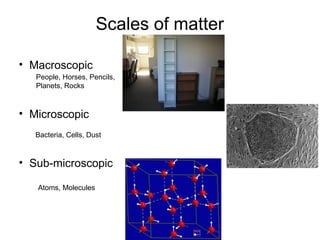
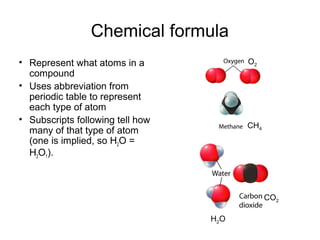
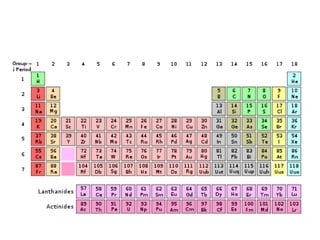
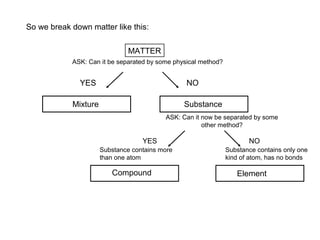
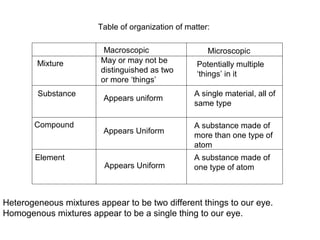
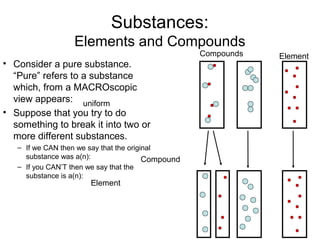
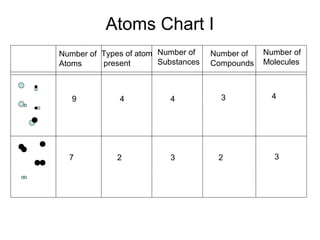
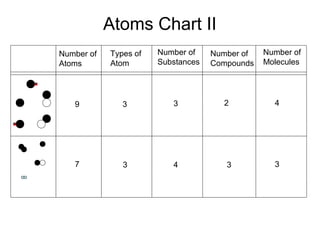
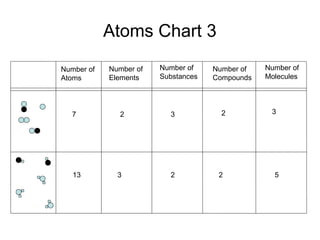
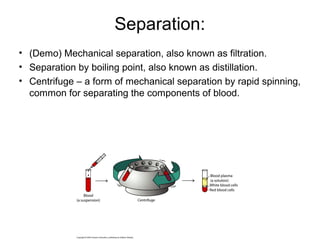
This document discusses the classification and properties of matter. It defines matter as anything that has mass and takes up space. Matter can be classified on the macroscopic, microscopic, and sub-microscopic scales. At the sub-microscopic scale, all matter is made up of atoms. The document discusses physical and chemical properties and changes. It also introduces mixtures, compounds, elements and discusses how matter can be classified and separated.