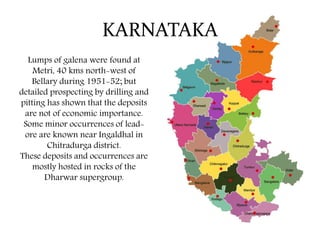
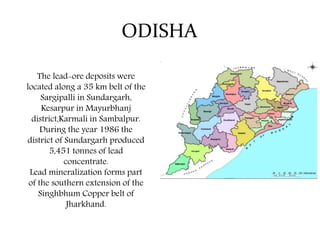
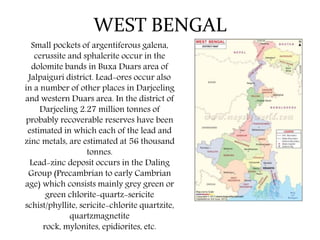
The document discusses lead deposits in India. It notes that 85% of India's lead deposits are located in Rajasthan, with major deposits found in Rampura-Agucha, Rajpura-Dariba, and Sindesar. It also lists lead deposits and occurrences in other states such as Andhra Pradesh, Jharkhand, Madhya Pradesh, Karnataka, Odisha, Gujarat, and West Bengal. The chief ores of lead discussed are galena, cerussite, and anglesite. Current uses of lead mentioned include lead-acid batteries, stained glass production, and fishing sinkers.