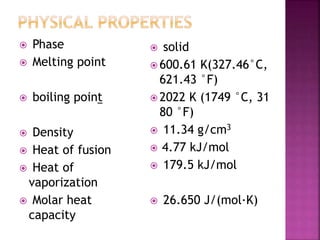
This document provides information about the chemical element lead. It lists lead's atomic number, group, period, electron configuration, physical properties such as melting point and density, common oxidation states, and ionization energies. It describes lead's historical and modern uses in products such as paint, pipes, batteries, and bullets, as well as its toxicity to humans when inhaled or ingested. Lead was widely used throughout history until its health effects were understood in the 20th century.


![ Atomic number
group, period
Block
Element category
standard atomic
weight
electron
configuration
electrons per
shell
82
group 14 (carbon
group), period 6
p-block
post-transition
metal
207.2(1)[1]
[Xe] 4f14 5d10 6s2
6p2
2, 8, 18, 32, 18, 4](https://image.slidesharecdn.com/lead-powerpoint-presentation-170903120907/85/Lead-powerpoint-presentation-4-320.jpg)