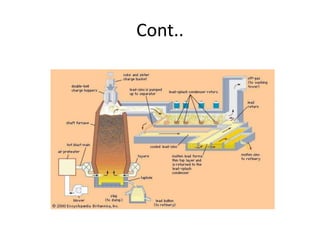
Lead is a soft, dense metal commonly used in batteries, paints, and other products. It is extracted from galena ore through processes like concentration, flotation, roasting, sintering, and smelting to produce molten lead bullion. Further refining removes impurities through processes like drossing and the Parkes process to produce 99-99.999% pure lead, which is then cast into blocks. Byproducts include sulfuric acid, slag, and air pollution, which require proper handling and disposal to prevent environmental contamination.