This document details the drug Lurasidone, branded as Latuda®, an atypical antipsychotic approved for treating schizophrenia in adults. The document outlines its chemical structure, mode of action, dosages, indications, and potential side effects, emphasizing its unique receptor-binding profile and lack of certain negative side effects typical of other antipsychotics. It also discusses the drug's advantages and disadvantages compared to existing medications and notes its expected rise in market sales as a leading atypical antipsychotic.

![1- Generic / INN Name : Lurasidone
2- Brand Name : Latuda®
3- Chemical Name :
(3aR,4S,7R,7aS)-2-[((1R,2R)-2-{[4-(1,2-benzisothiazol-3-yl)-
piperazin-1-yl]methyl}cyclohexyl)methyl]hexahydro-1H-4,7-
methanisoindol-1,3-dione .
4- CASR # : 367514-88-3
5- Innovative Company & ( Country ) :
Dainippon Sumitomo Pharma Co., Ltd.
Which is a pharmaceutical company based in Japan. Its headquarters are
in Chuo-ku, Osaka.
Current Manufacturer is Sunovion Pharmaceuticals.
6- Chemical Structure :](https://image.slidesharecdn.com/latuda-120522052924-phpapp02/85/Latuda-Lurasidone-2-320.jpg)


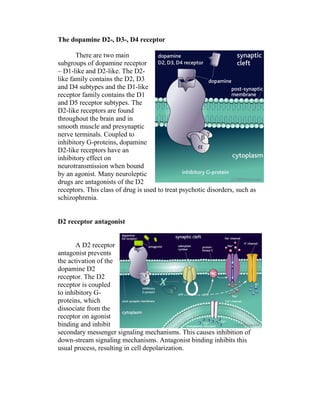
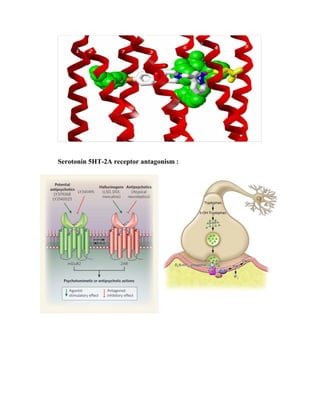
![13- Pharmacophoric group :
(1R,2S,6R,7S)-4-{[(1R,2R)-2-{[4-(1,2-Benzothiazol-3-yl)-1-
piperazinyl]methyl}cyclohexyl]methyl}-4-azatricyclo[5.2.1.02,6]decane-
3,5-dione , structure shows 6 stereocenters that have major influence on
its action as atypical antipsychotic drug .
14- Expected ADME Profile :
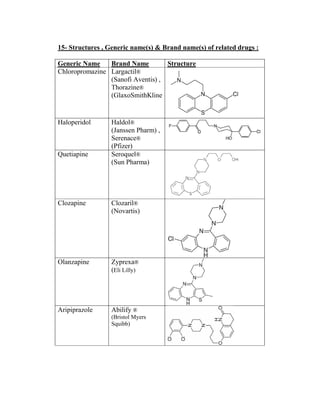
- Absorption
Lurasidone is absorbed and reaches highest concentration in 1-3
hours. Nine to 19 percent of oral
administered dose is absorbed into the system. Its level is enhanced with
food consumption.
Cmax and AUC are increased by 3-times and 2-times, respectively, in the
presence of food.
- Distribution
98.8% of Lurasidone molecules is bound to plasma protein .
- Metabolism
Lurasidone is metabolized by CYP3A4 via oxidative N-
dealkylation, hydroxylation and S oxidation.
Major metabolites include two active and two non-active forms.
- Elimination
80% is excreted in feces and 9% in urine.](https://image.slidesharecdn.com/latuda-120522052924-phpapp02/85/Latuda-Lurasidone-8-320.jpg)