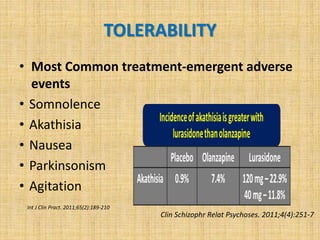
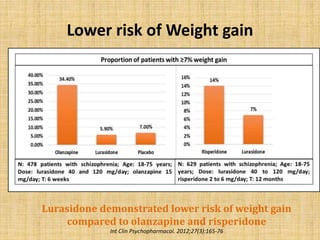
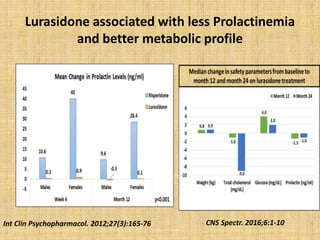
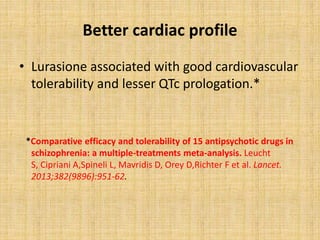
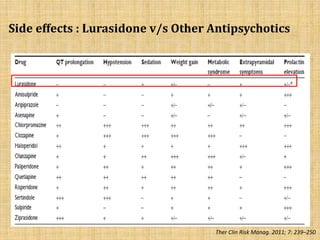
Lurasidone is a newer second-generation antipsychotic drug that is a full antagonist at dopamine D2 and serotonin 5HT2A receptors. It also has antagonist effects at 5HT7 receptors and is a partial agonist at 5HT1A receptors. Lurasidone has minimal affinity for receptors like alpha1, 5HT2C, histamine H1, and muscarinic M1, which predicts a lower risk of side effects like orthostatic hypotension, weight gain, and anticholinergic effects. Lurasidone has been approved by the FDA for the treatment of schizophrenia and bipolar depression.