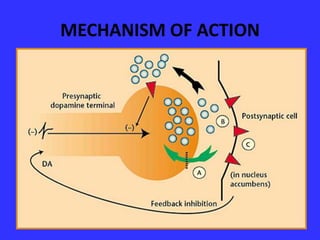
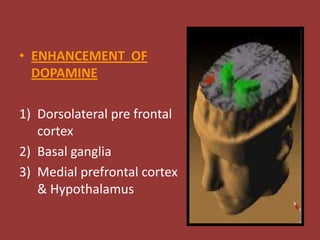
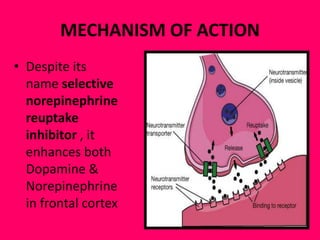
This document provides information on drug treatments for ADHD, including stimulant drugs like methylphenidate and dextroamphetamines, and non-stimulant drugs like atomoxetine, bupropion, and venlafaxine. It discusses the history, mechanism of action, brands/forms, dosage, side effects, abuse potential, and drug interactions of methylphenidate and atomoxetine in detail. Methylphenidate enhances dopamine levels in the brain's reward pathway and is a schedule II drug with abuse potential when crushed/injected. Atomoxetine is a selective norepinephrine reuptake inhibitor and may have less abuse potential than stimulants.



















![REFERENCES
1] Kaplan & Sadock’s Comprehensive
Textbook Of Psychiatry
2] Stephen Stahl's
Essential Psychopharmacology
3] Stephen Stahl’s
Prescribers guide
4] The Maudsley’s Prescribing
Guidelines](https://image.slidesharecdn.com/1-140113135354-phpapp01/85/atomoxetine-methylphenidilate-29-320.jpg)