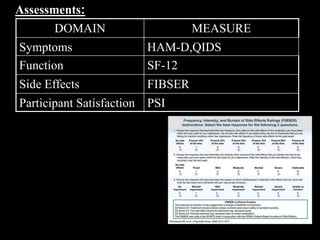
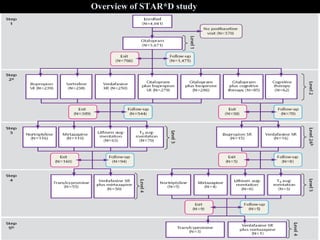
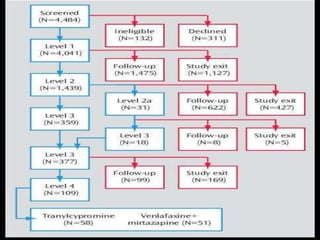
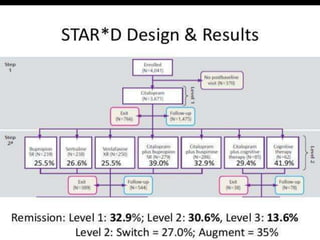
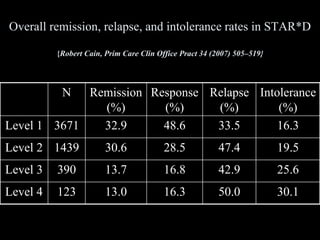
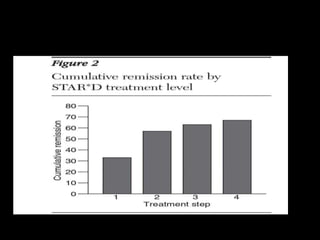
The STAR*D trial was a large, multi-center study that examined the effectiveness of different treatment options for patients with unipolar depression who did not achieve remission with an initial antidepressant. Over 4,000 outpatients were treated across four levels of sequentially increasing treatment intensity. The study found that after two treatment steps, around 67% of patients achieved remission, but relapse rates were high. Patients with more severe and chronic illness required more treatment steps to achieve remission. While the study provided important real-world data on treating depression, it had some limitations like lack of placebo groups and small sample sizes in later levels.
![STAR*D
Sequenced Treatment Alternatives To Relieve Depression
-Dr.maithri[1st yr pg psychiatry]
Moderator-Dr.Sai kiran Pasupula
Assistant Professor](https://image.slidesharecdn.com/star-drevisedfinal-200617154048/75/Star-d-revised-final-1-2048.jpg)















![Level 2A
Level 2
Level 3
Level 4
Level 1
STAR*D Treatment Algorithm
• Initial treatment: Citalopram
• Switch to Bup[SR] / Ven[ER] / Sert / CT
• Augment with Bup[SR] / Bus / CT
• Only for those receiving CT, Switch to Bup /
Ven if exiting level 2
• Switch to Mirt / Nortryp
• Augment with Lithium / T3
• Switch to Tranylcypromine / Mirt + Ven[ER]](https://image.slidesharecdn.com/star-drevisedfinal-200617154048/85/Star-d-revised-final-17-320.jpg)