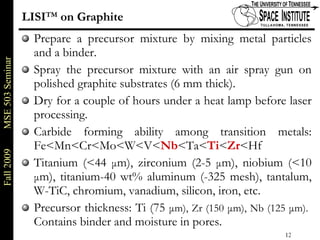
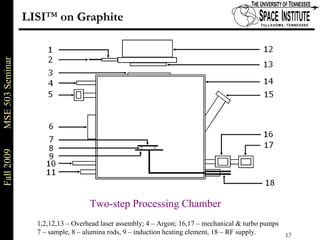
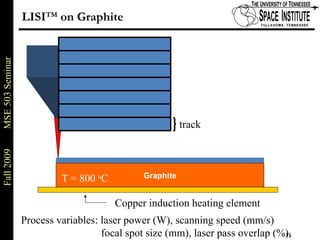
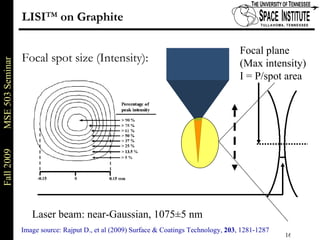
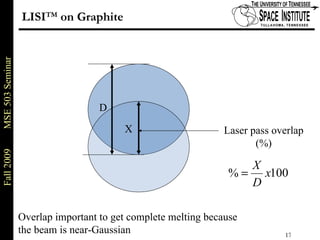
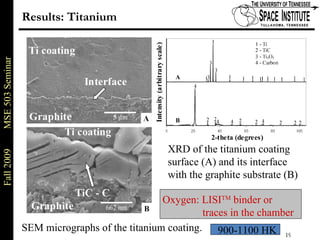
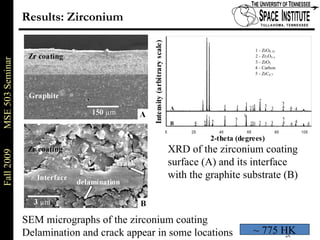
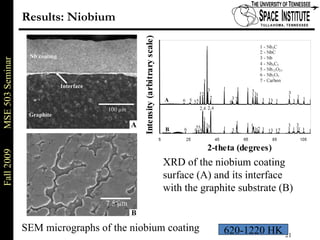
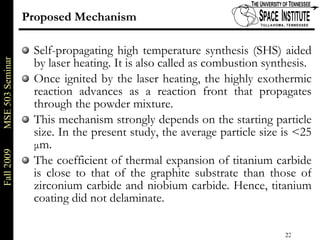
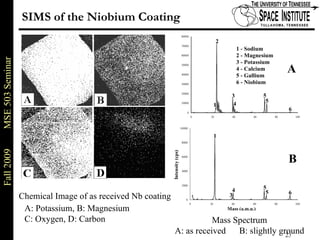
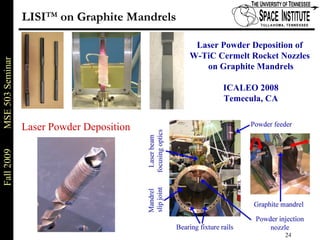
Deepak Rajput presented research on laser processing of graphite. Titanium, zirconium, and niobium coatings were deposited on graphite substrates using the LISI TM process. Characterization found the coatings were fully dense and crack-free with carbide phases formed at the interfaces. A proposed mechanism is laser-assisted self-propagating high temperature synthesis due to the exothermic reactions. Future work includes heat treatment, advanced characterization, and publishing the results.
![Laser Processing on Graphite MSE 503 Seminar - Fall 2009 08-27-2009 CLA Conference Room, UT Space Institute, Tullahoma, TN - 37388, USA Deepak Rajput Graduate Research Assistant Center for Laser Applications University of Tennessee Space Institute Tullahoma, Tennessee 37388-9700 Email: [email_address] Web: http:// drajput.com](https://image.slidesharecdn.com/graphite-091217183934-phpapp02/75/Laser-Processing-on-Graphite-1-2048.jpg)