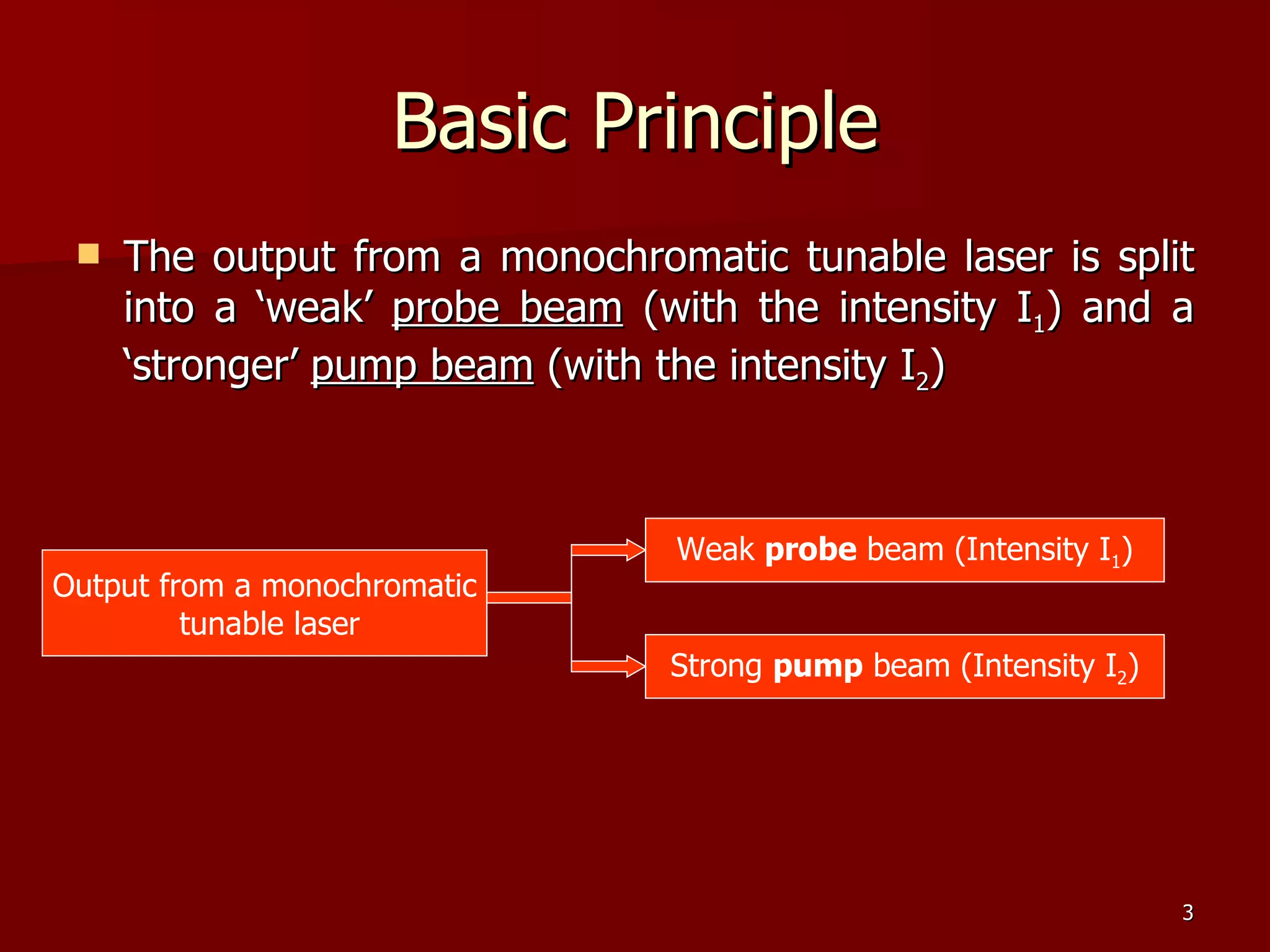
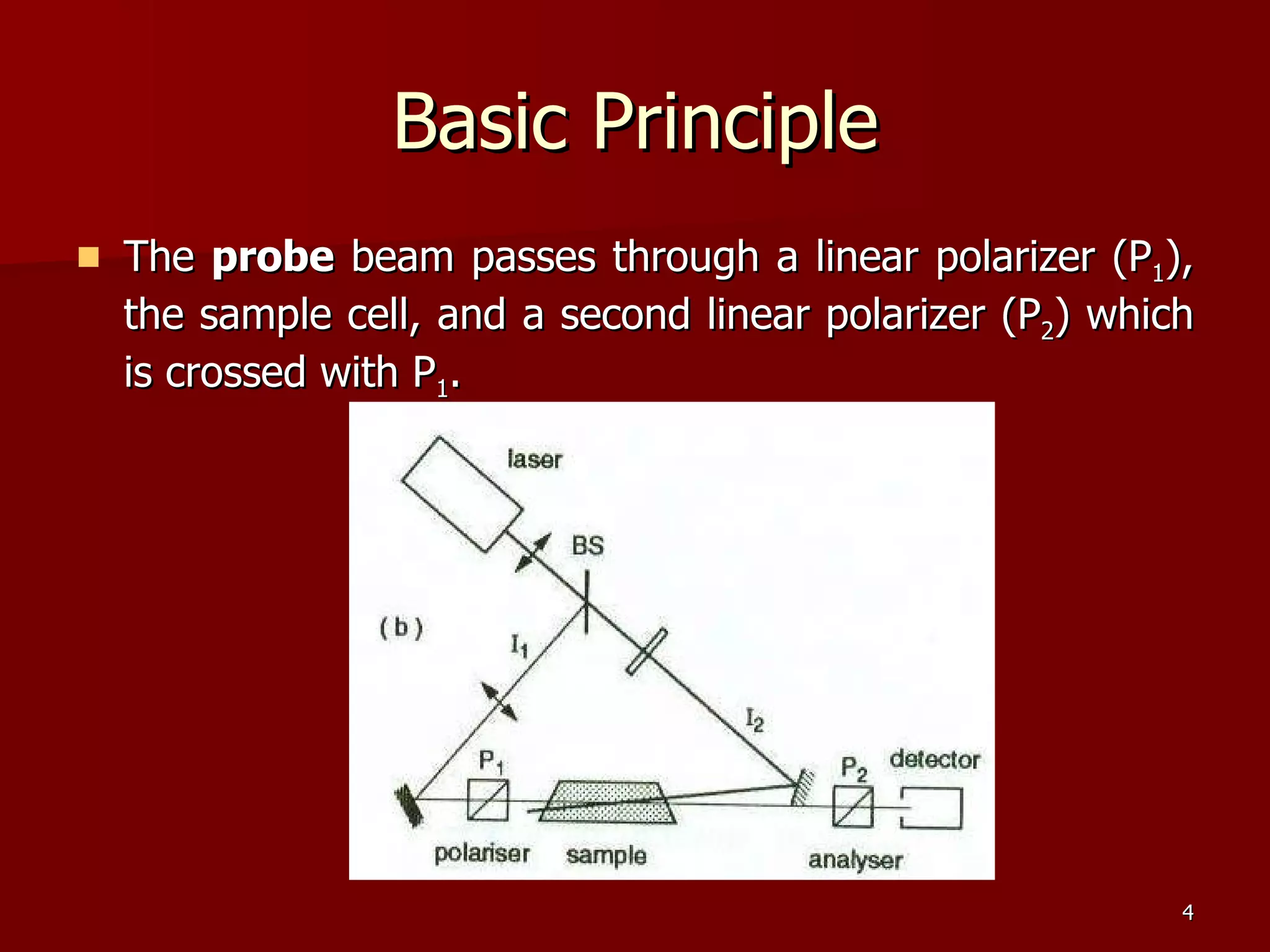
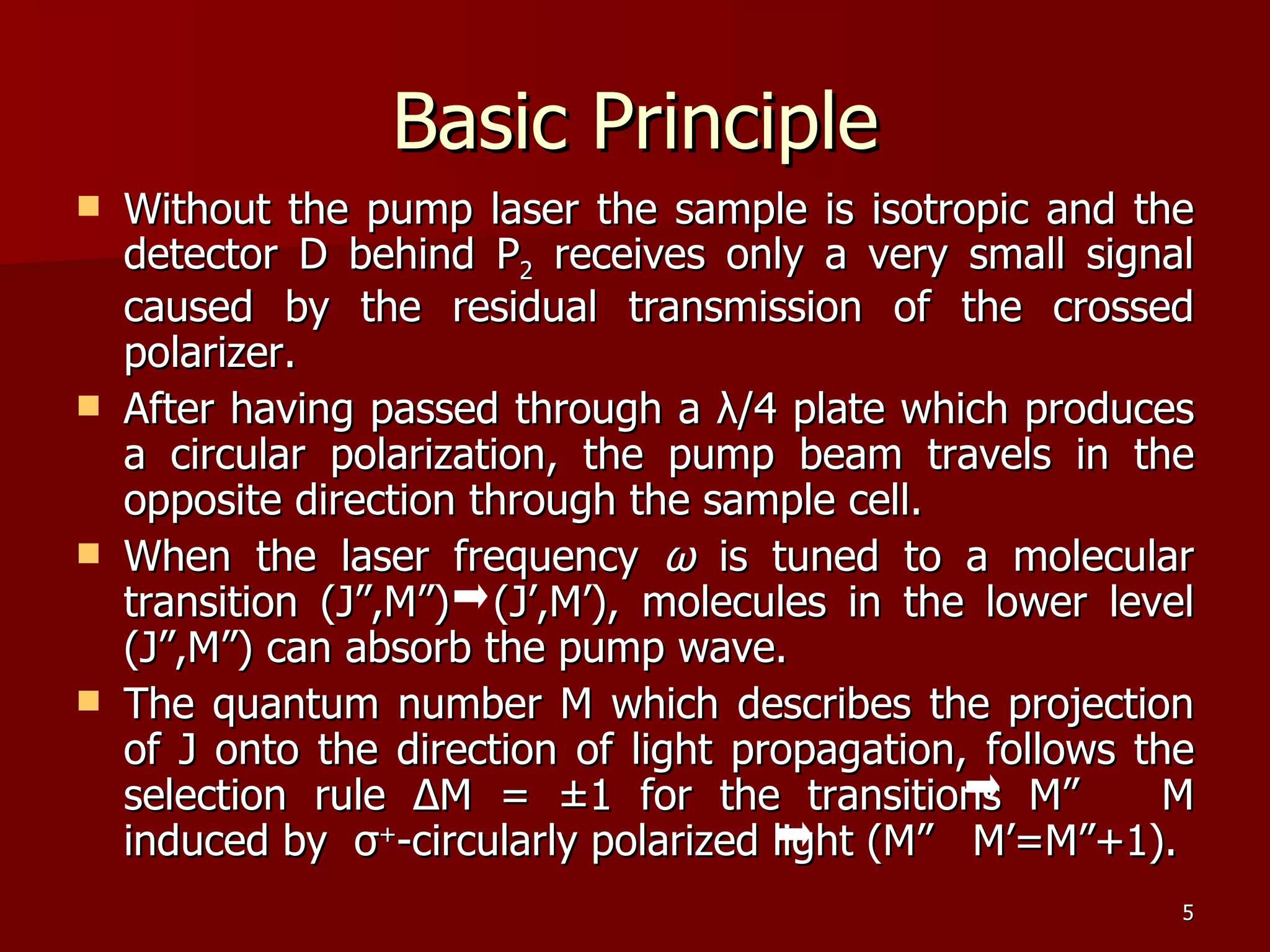
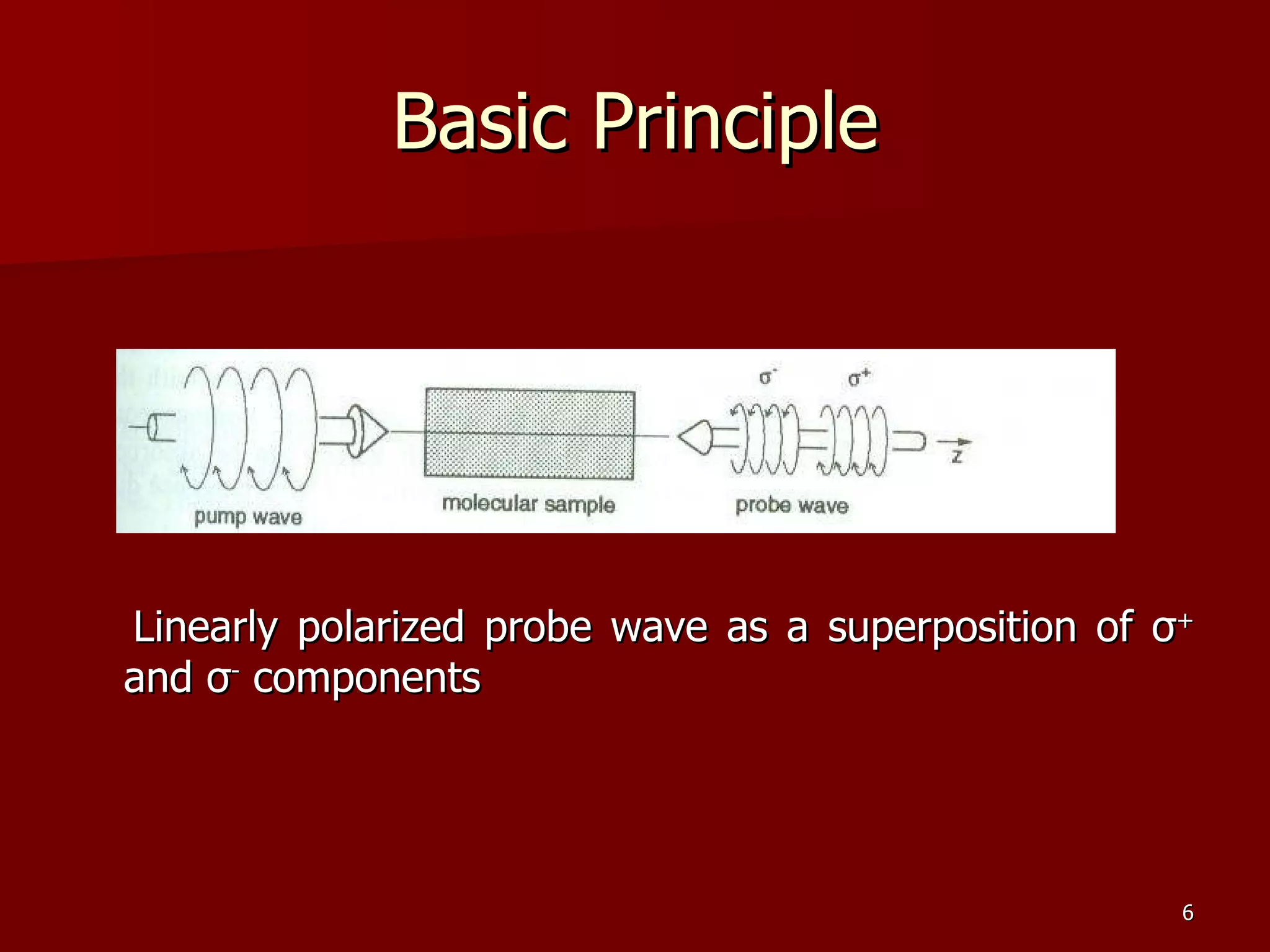
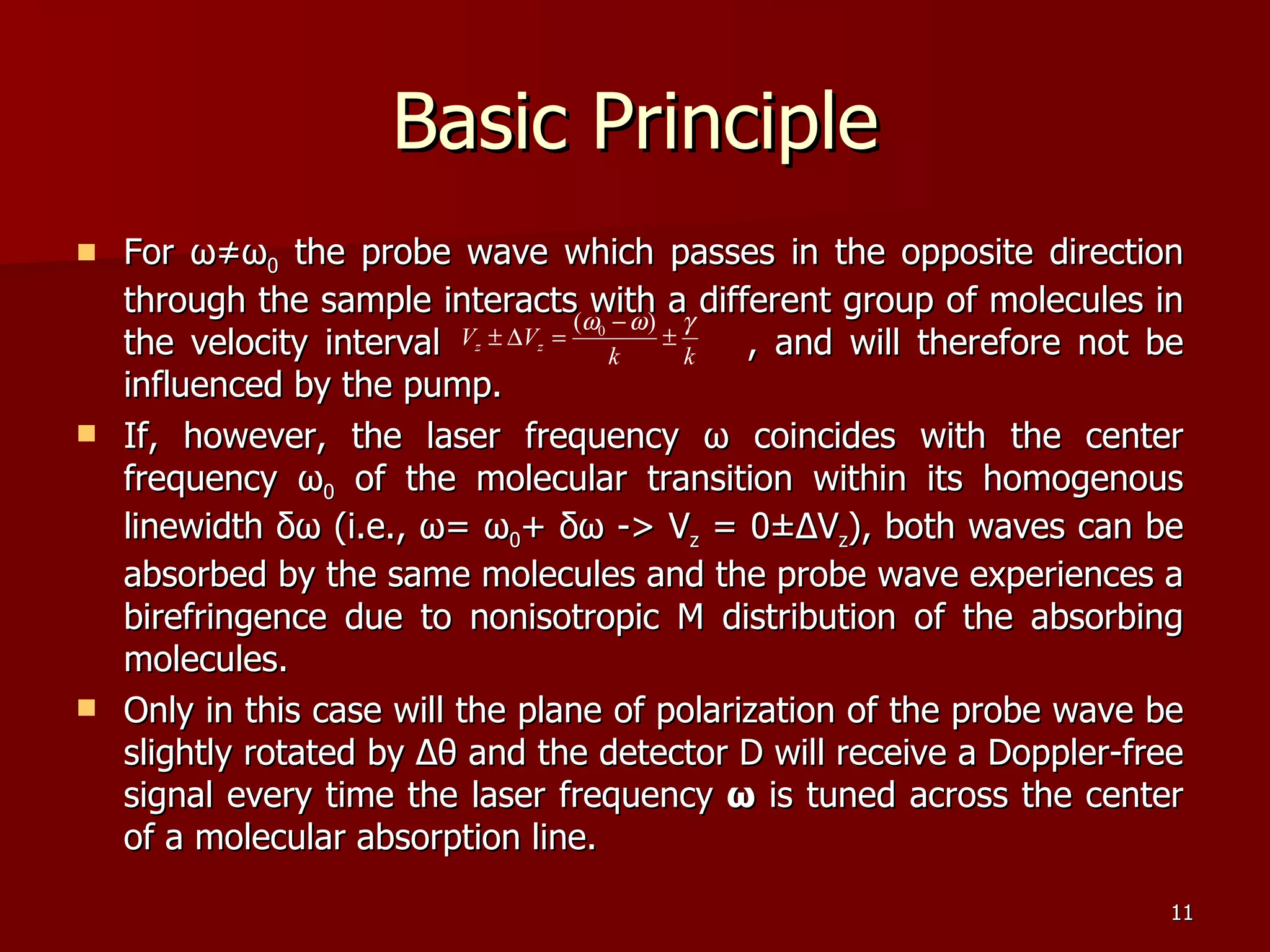
The seminar by Deepak Rajput discusses polarization spectroscopy, a technique utilizing the polarization properties of light to measure molecular transitions. It explains the process involving weak and strong beams, the role of a linear polarizer, and the effects of saturation on molecular sublevels leading to birefringence. The concept allows for detecting molecular interactions without the need for a magnetic field by analyzing the rotation of the probe wave's polarization through an anisotropic sample.
![Polarization Spectroscopy Seminar by Deepak Rajput PHYS 605 Advanced Topics: Laser Spectroscopy July 10, 2007 Center for Laser Applications University of Tennessee Space Institute Tullahoma, TN 37388 Email: [email_address] Web: http://drajput.com](https://image.slidesharecdn.com/polarizationspectroscopy-091218111842-phpapp02/75/Polarization-Spectroscopy-1-2048.jpg)