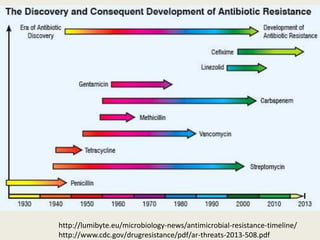
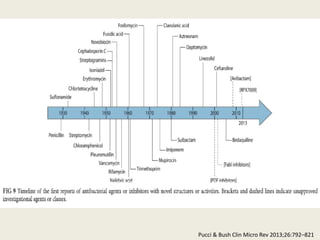
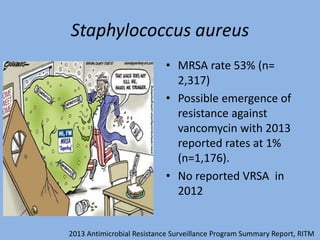
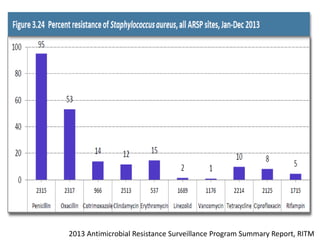
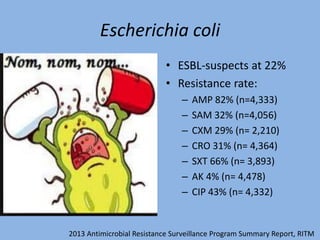
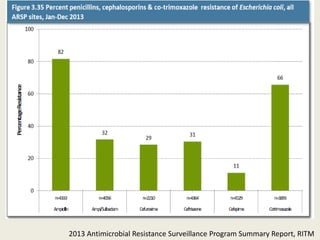
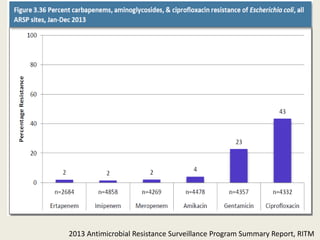
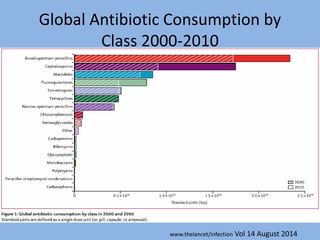
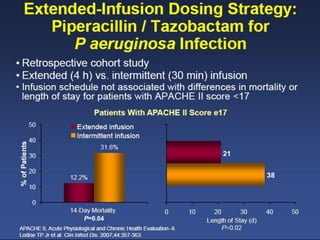
This document discusses the problem of increasing antimicrobial resistance and outlines strategies for antimicrobial stewardship programs. It recognizes antimicrobial resistance as a serious global problem that requires immediate action. Antimicrobial stewardship is defined as optimizing antibiotic use through selecting appropriate treatment duration, dose, and spectrum of coverage. The document recommends establishing multidisciplinary stewardship teams and implementing interventions like guidelines, audit and feedback, and streamlining of therapy to improve antibiotic use and slow the development of resistance. Physicians are identified as key players that can help address the problem through their antibiotic prescribing practices.