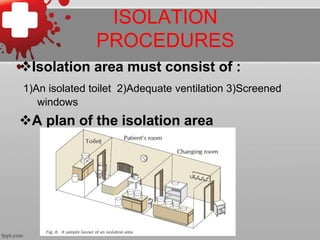
The document discusses the prevention of Ebola virus infection and associated challenges. It outlines people at risk of infection, case definitions, laboratory tests for diagnosis, screening procedures at airports, isolation and treatment protocols, contact tracing, precautions for healthcare workers, waste management procedures, vaccine candidates, and post-exposure prophylaxis. It identifies challenges to prevention as weak health systems, cultural and economic factors, lack of international cooperation, and technical difficulties in research and developing effective treatments.