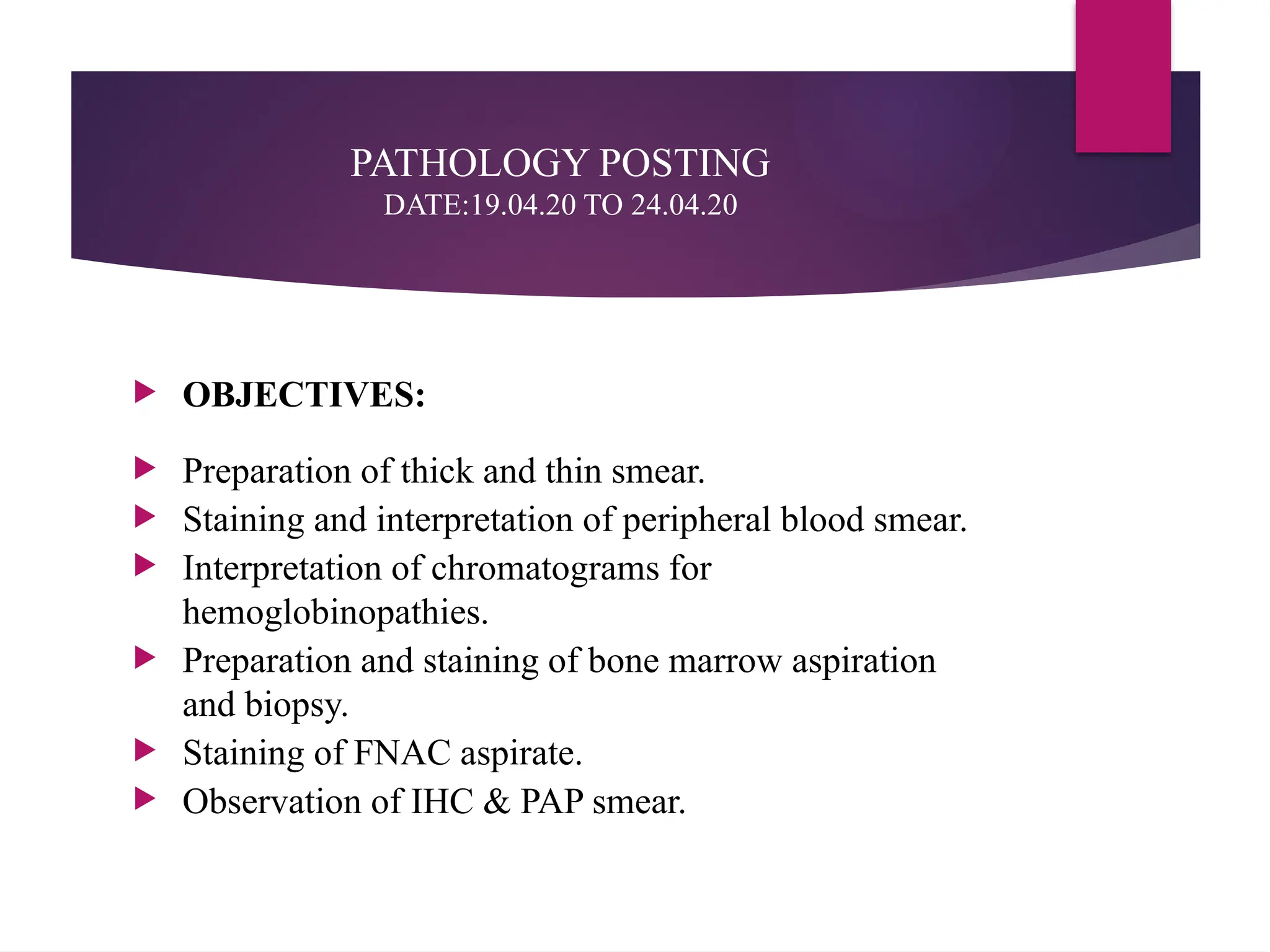
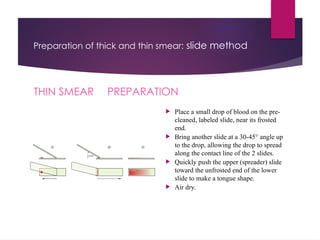
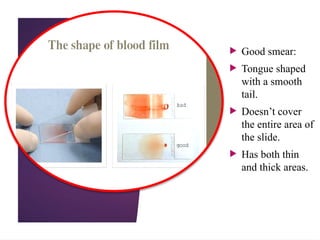
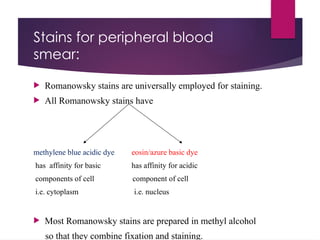
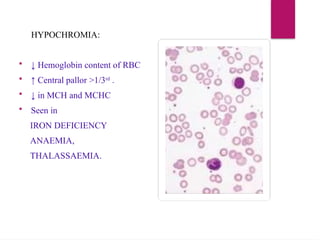
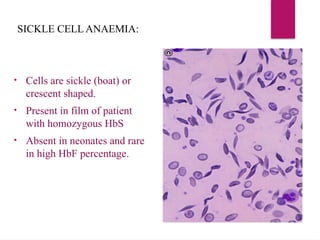
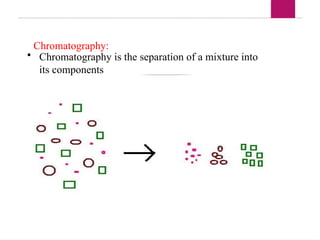
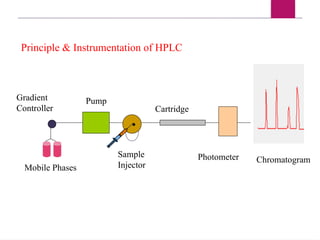
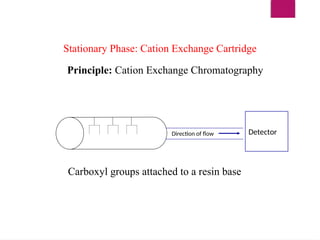
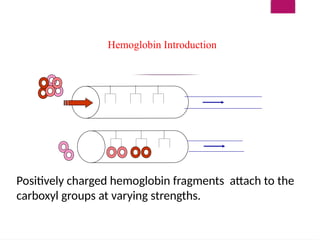
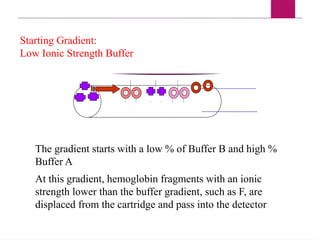
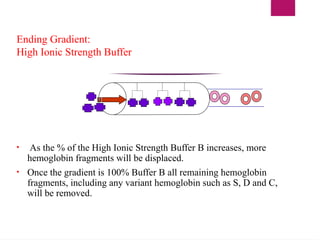
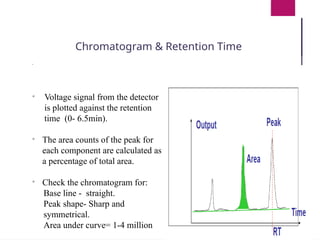
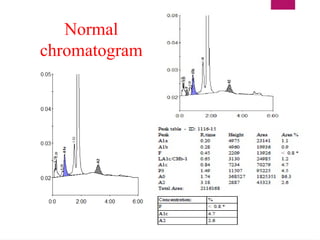
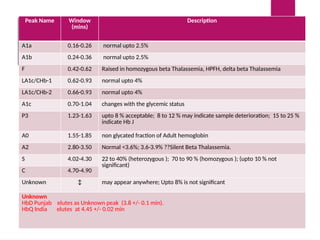
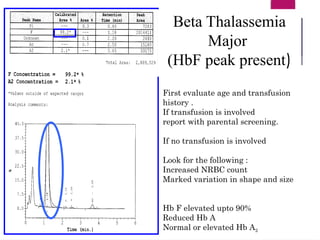
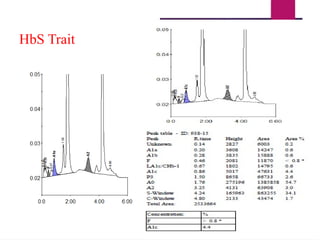
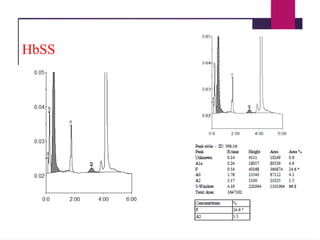
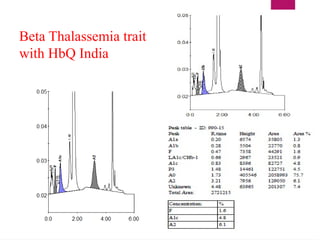
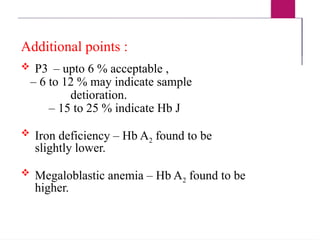
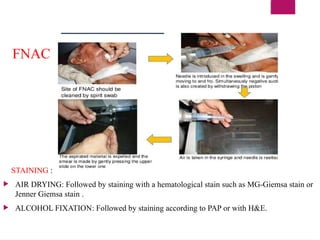
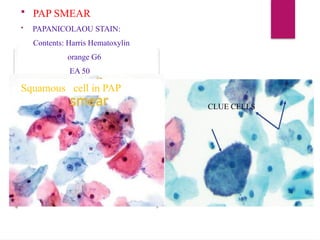
The document outlines procedures for preparing and interpreting blood smears, including thick and thin smears, fixation, and staining techniques using various Romanowsky stains. It details the identification of cellular elements in peripheral blood, bone marrow aspiration, and techniques for interpreting hemoglobinopathies through chromatography. Additionally, it covers methodologies for pap smears and immunohistochemistry, highlighting the importance of accurate sample preparation and analysis in hematology.