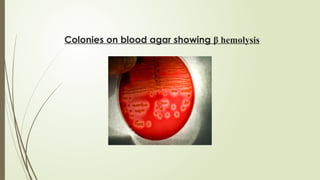
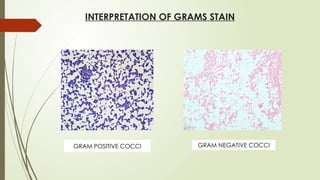
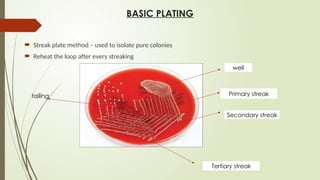
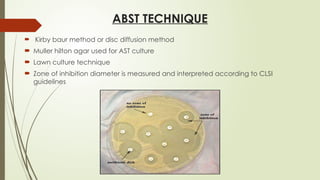
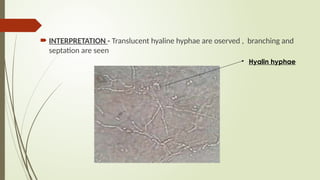
The document outlines microbiology laboratory procedures for specimen collection and processing, including blood, urine, pus, cerebrospinal fluid (CSF), and stool. It details various techniques such as Gram staining, automated blood culture systems (Bactec and Vitek), and molecular methods for diagnosing infectious diseases. Additional information covers culture media used, antibiotic susceptibility testing, and identification of pathogens through biochemical tests.