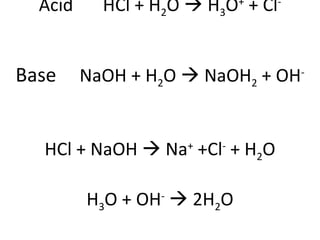
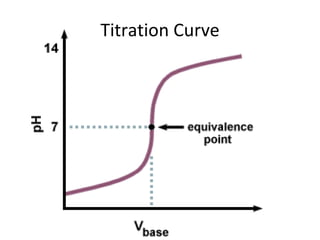
The document discusses titration as a method to determine unknown concentrations of acids and bases. Titration involves slowly adding a base to an acidic solution until the pH reaches 7.00, indicating neutralization. The concentration of the base and volumes of the acid and base added can be used to calculate the concentration of the original acid using the titration equation. Indicator solutions that change color between acid and base are used to pinpoint the endpoint of neutralization.