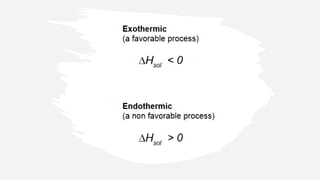
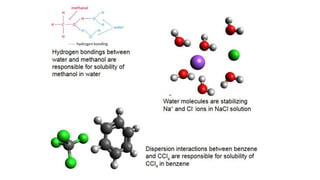
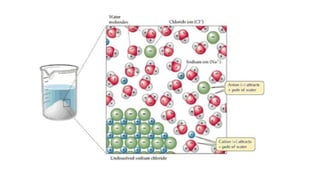
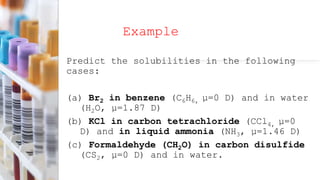
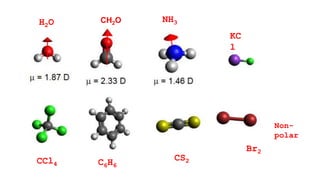
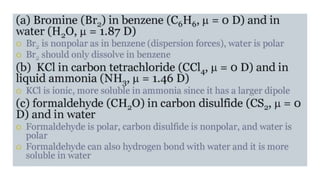
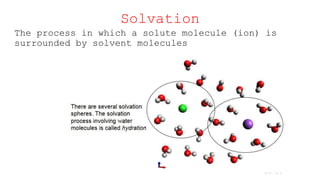
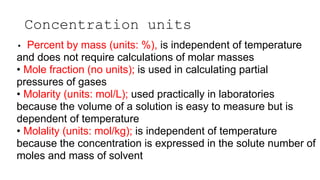
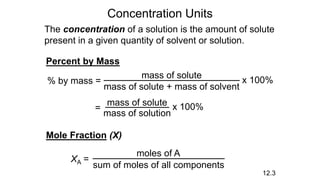
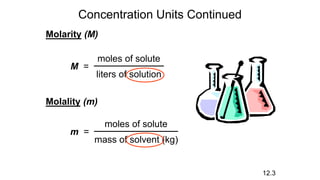
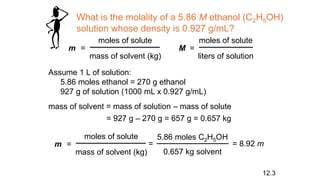
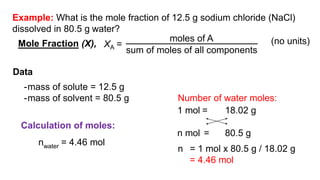
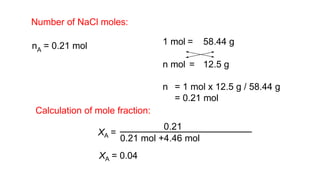
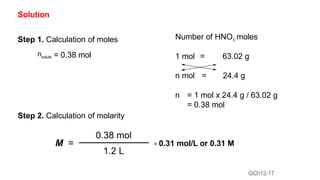
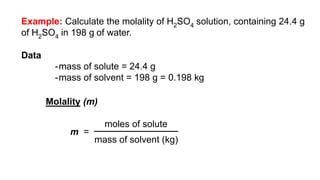
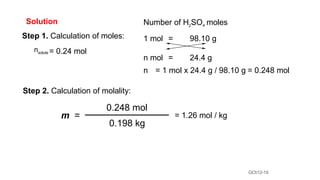
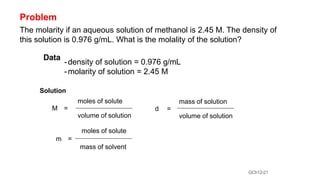
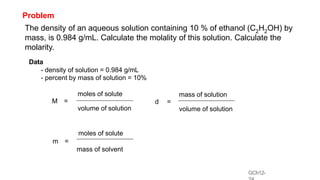
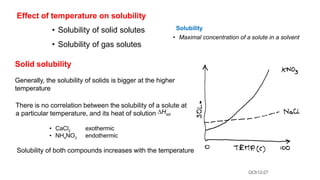
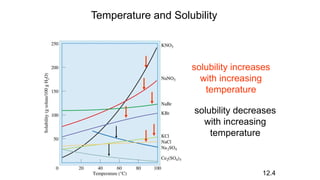
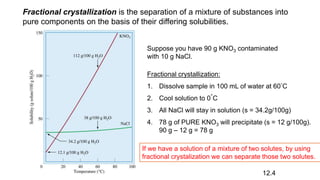
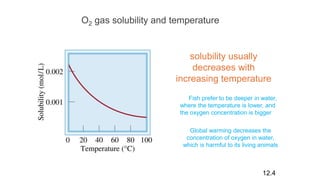
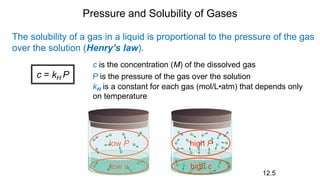
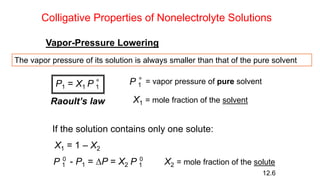
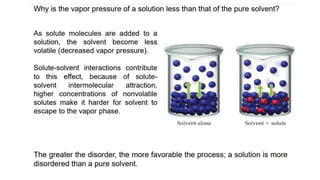
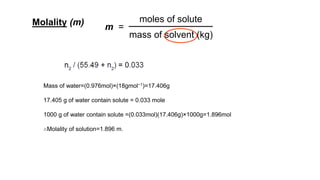
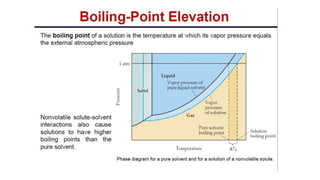
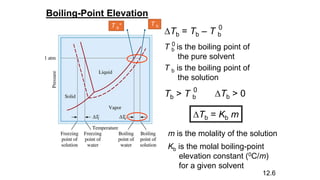
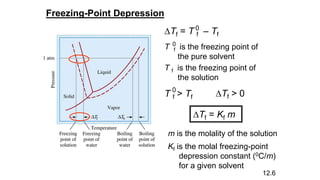
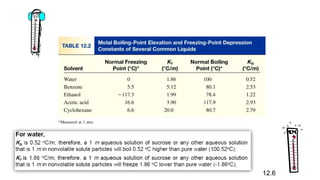
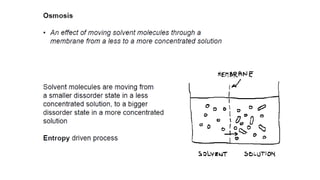
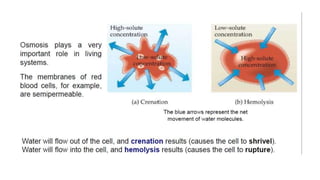
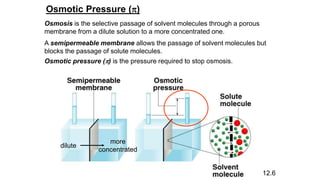
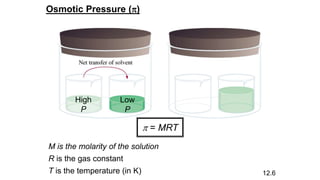
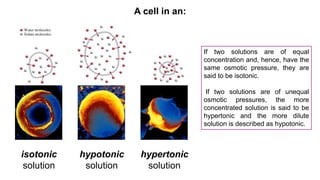
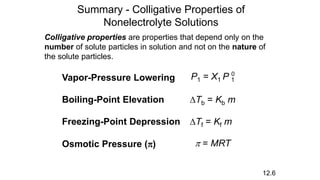
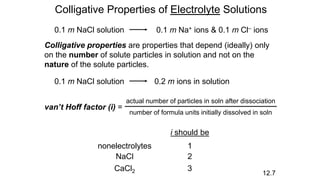
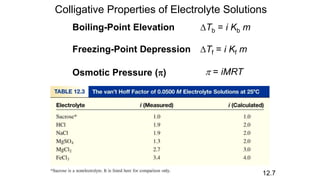
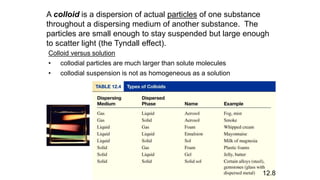
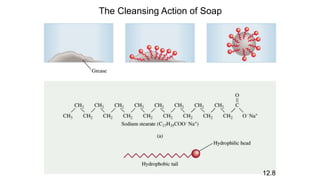
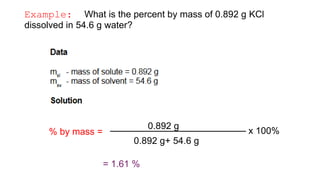This document provides an overview of CHE 106 Chemistry course. The purpose of the course is to build a solid foundation of basic chemical concepts and principles for students and show examples of chemistry's important role in daily life to get students interested in chemistry. The course will use the textbook Chemistry by Chang R. and recommended supporting textbooks. It aims to develop students' understanding of fundamental chemical concepts and principles and inspire interest in chemistry through examples of its relevance to everyday life.