The document discusses membrane transport and the membrane potential. It covers several key topics:
1. The plasma membrane acts as a selective barrier between the intracellular and extracellular environments. Transport across the membrane can occur through passive or active mechanisms.
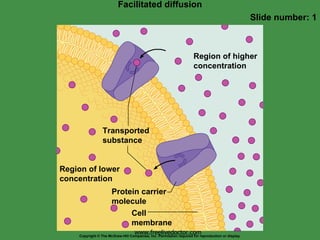
2. Passive transport mechanisms include diffusion, facilitated diffusion, and osmosis. Diffusion is the random movement of particles down a concentration gradient without energy input.
3. Active transport moves particles against a concentration gradient and requires energy in the form of ATP hydrolysis. The sodium-potassium pump is an example of primary active transport.
4. Transport proteins act as carriers to facilitate the movement of specific molecules or ions across the membrane. Carrier-mediated transport