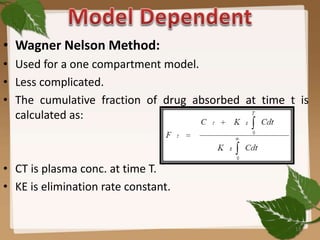
This document discusses in vitro-in vivo correlation (IVIVC), which is a mathematical model that can estimate in vivo behavior and drug absorption from in vitro dissolution testing. It describes the different levels of IVIVC correlation from A to D, with Level A being the highest level where in vitro and in vivo curves are superimposable. Key aspects covered include the role of biopharmaceutics in IVIVC, methods for establishing correlations like deconvolution, and uses of IVIVC in drug development and regulation.