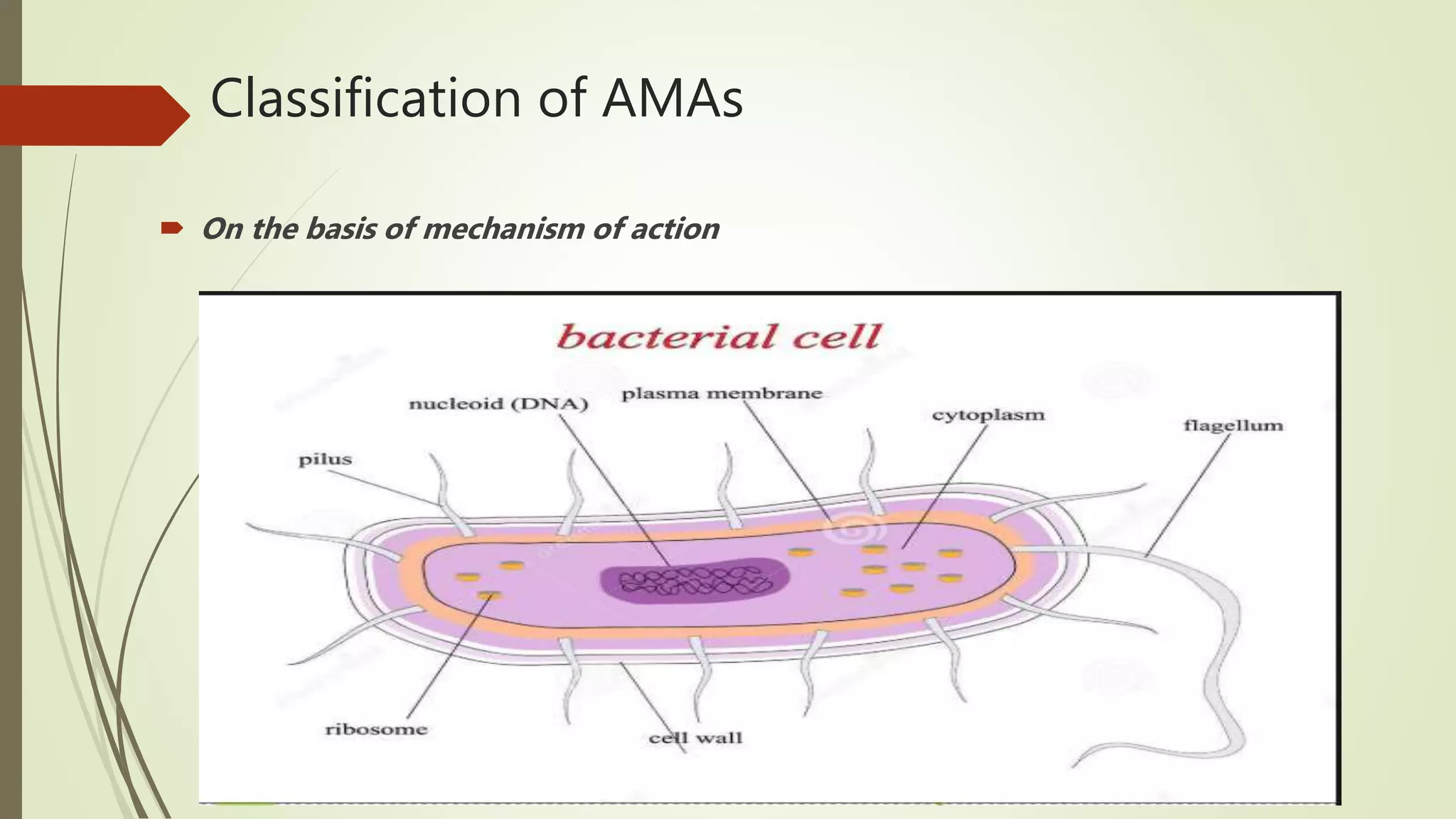
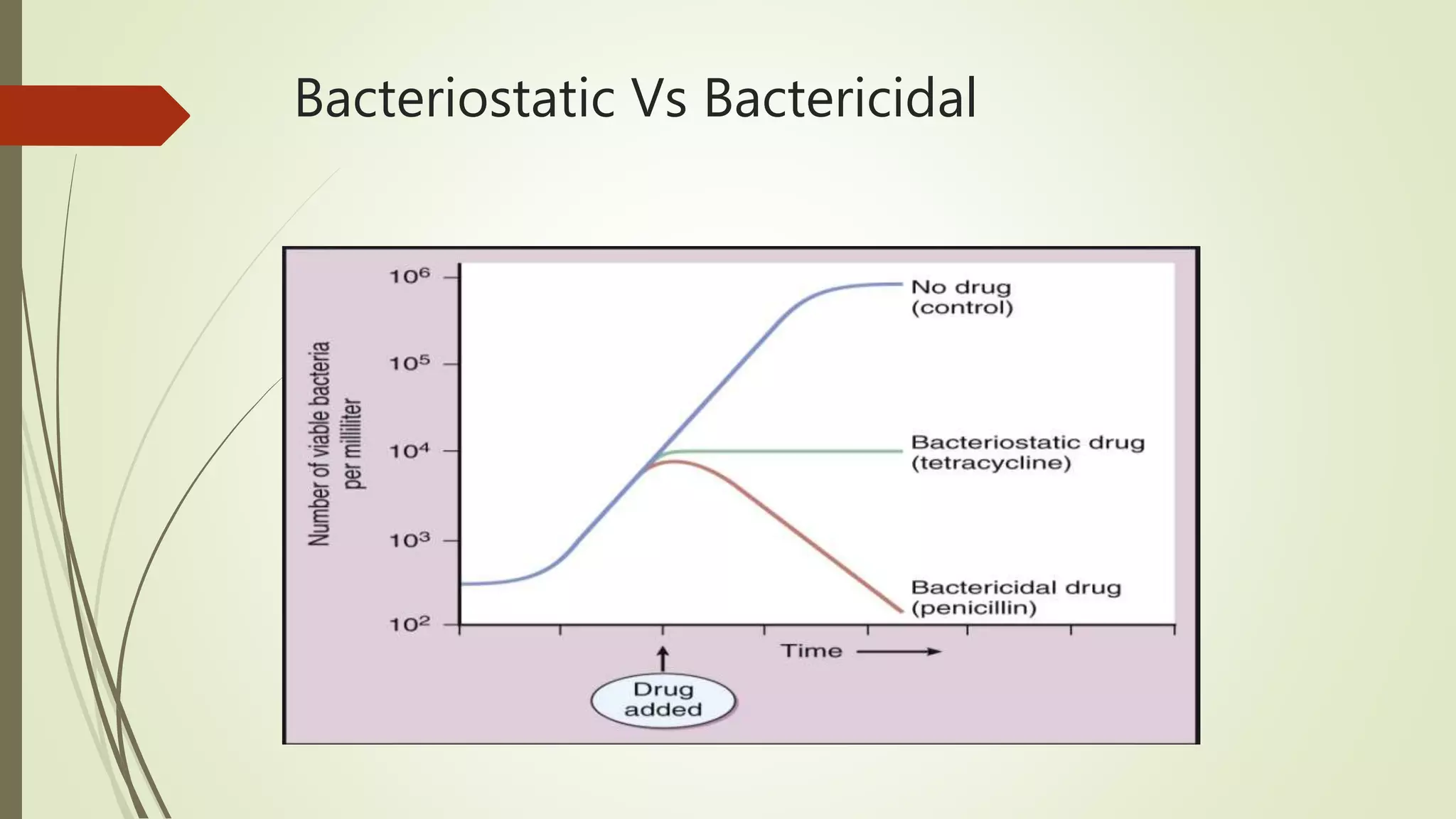
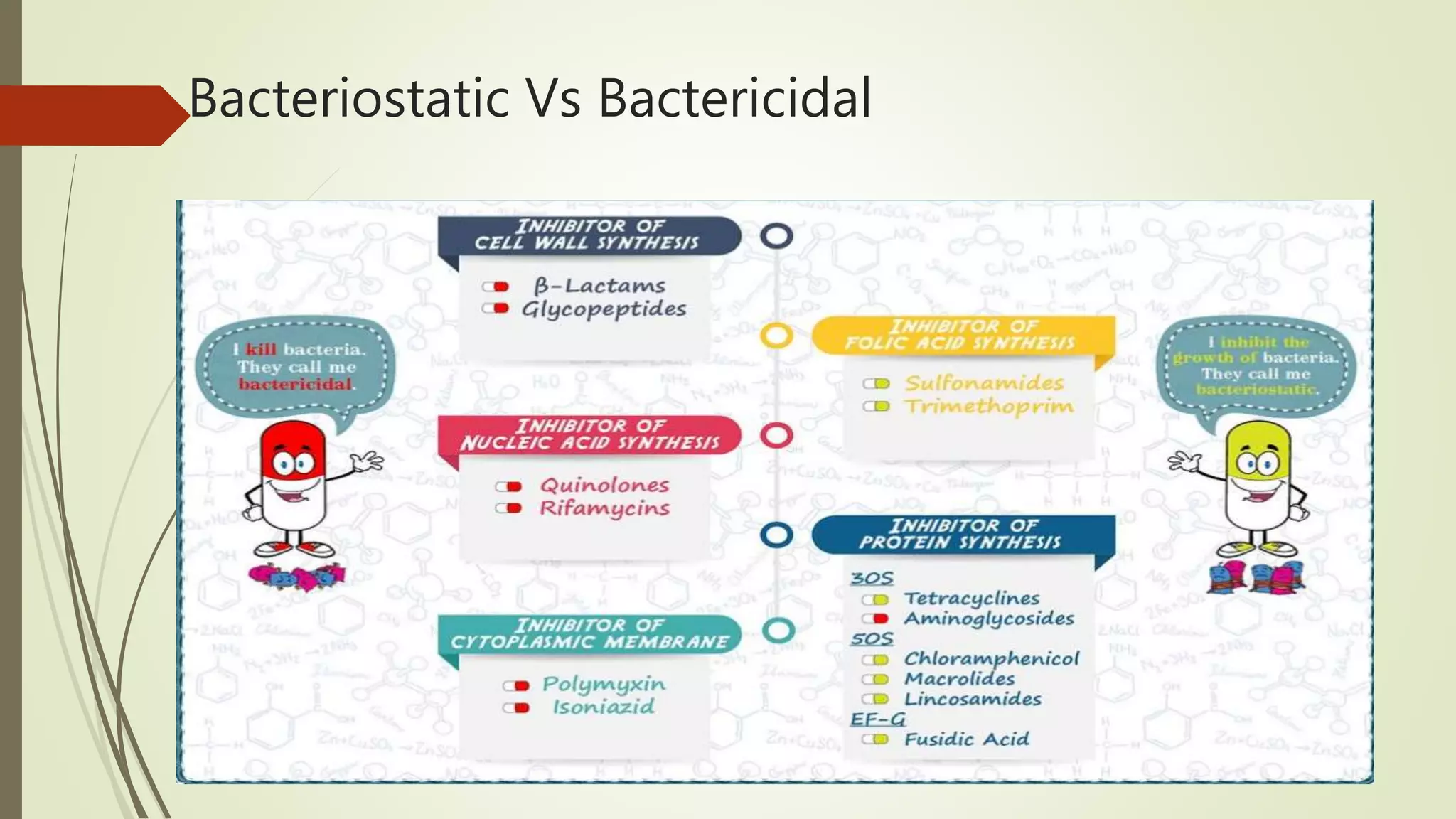
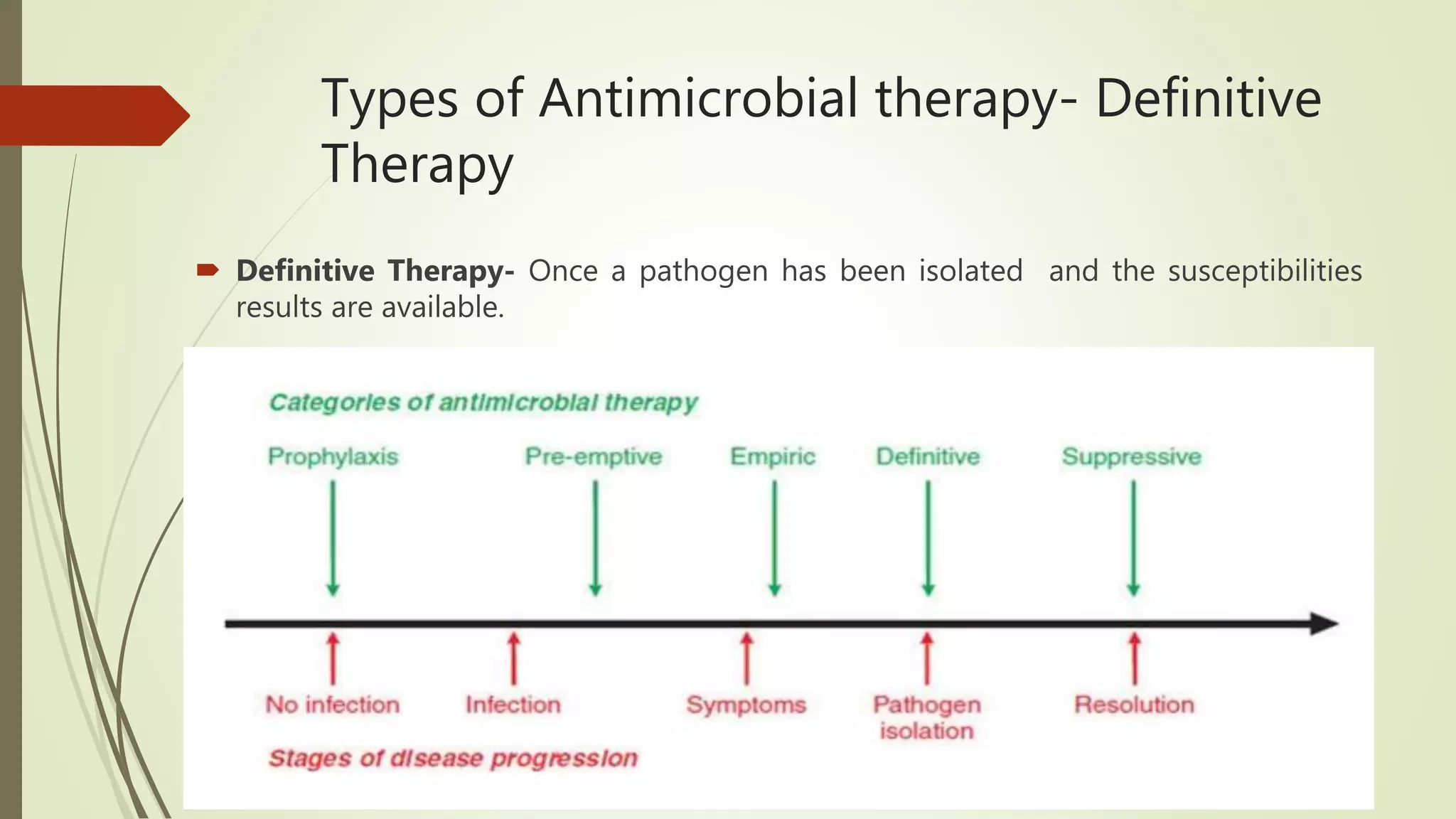
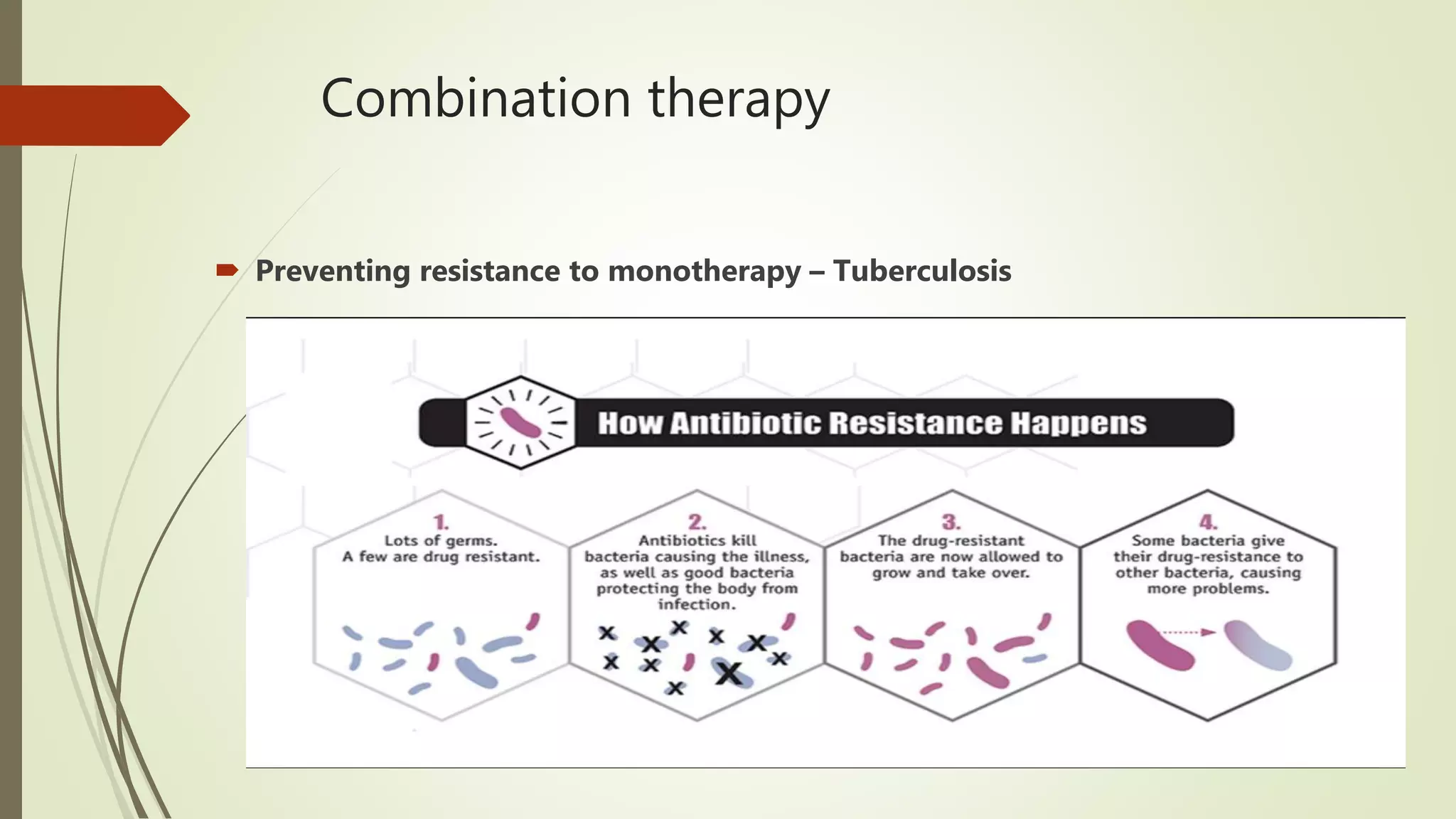
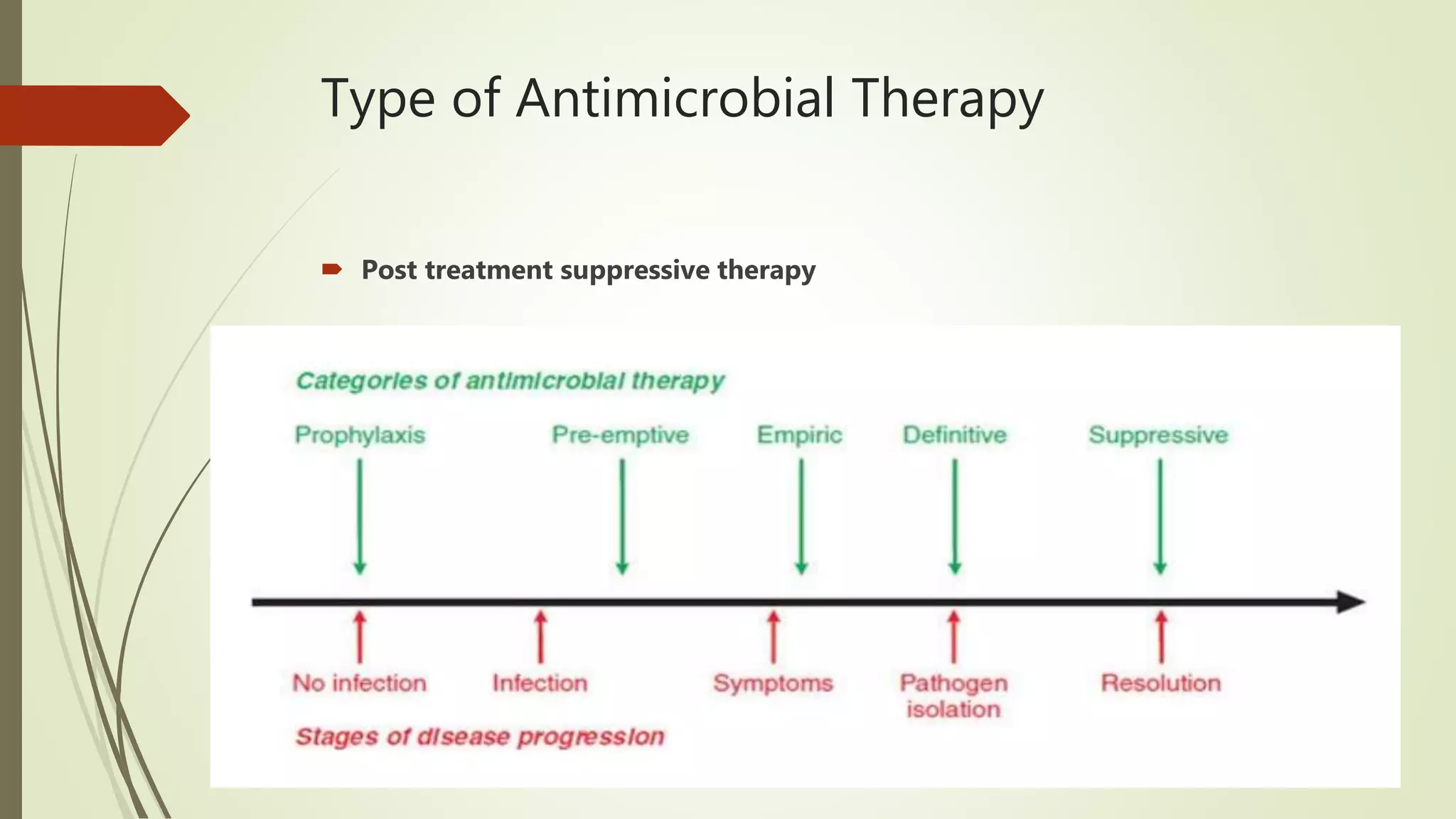
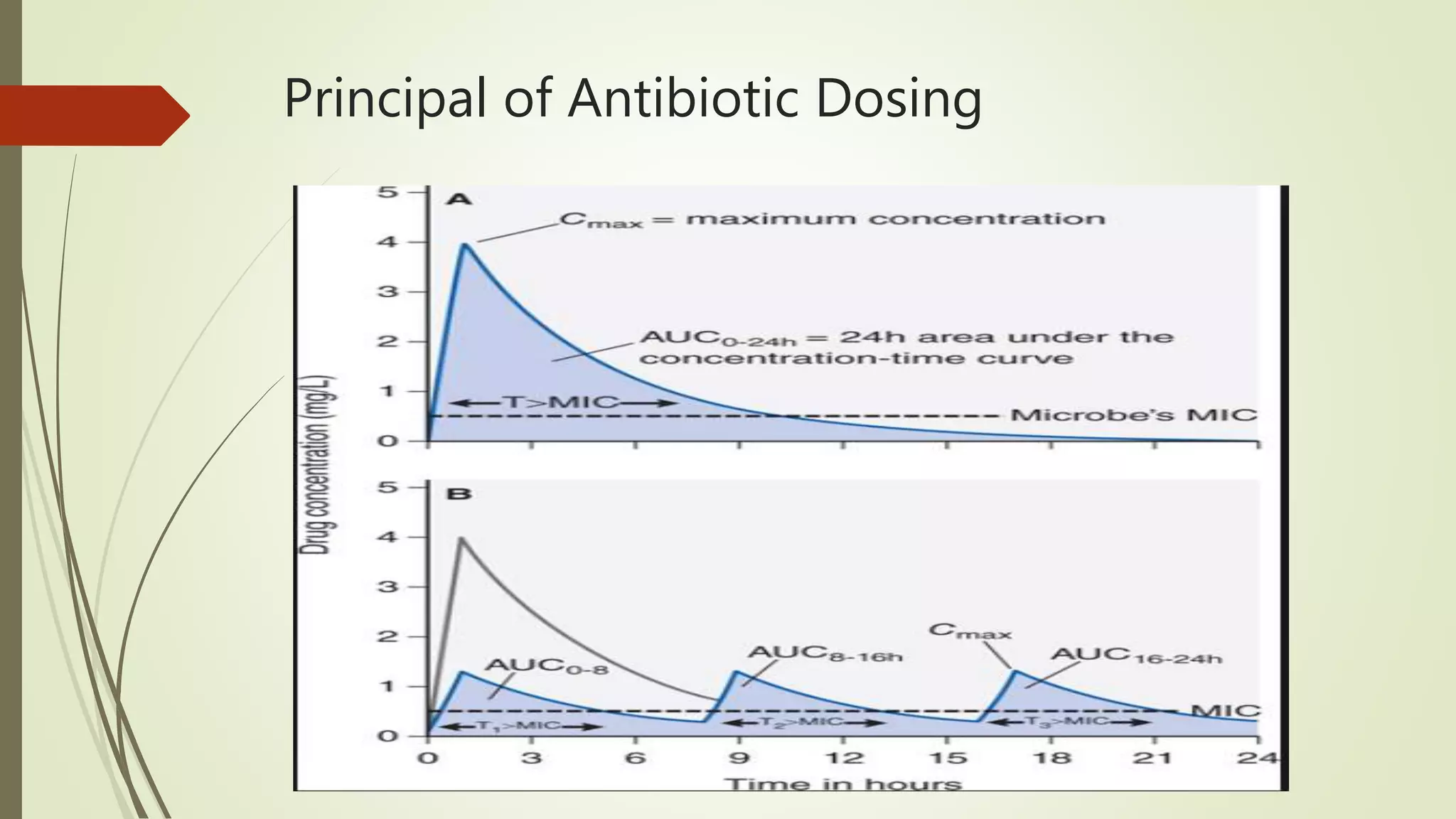
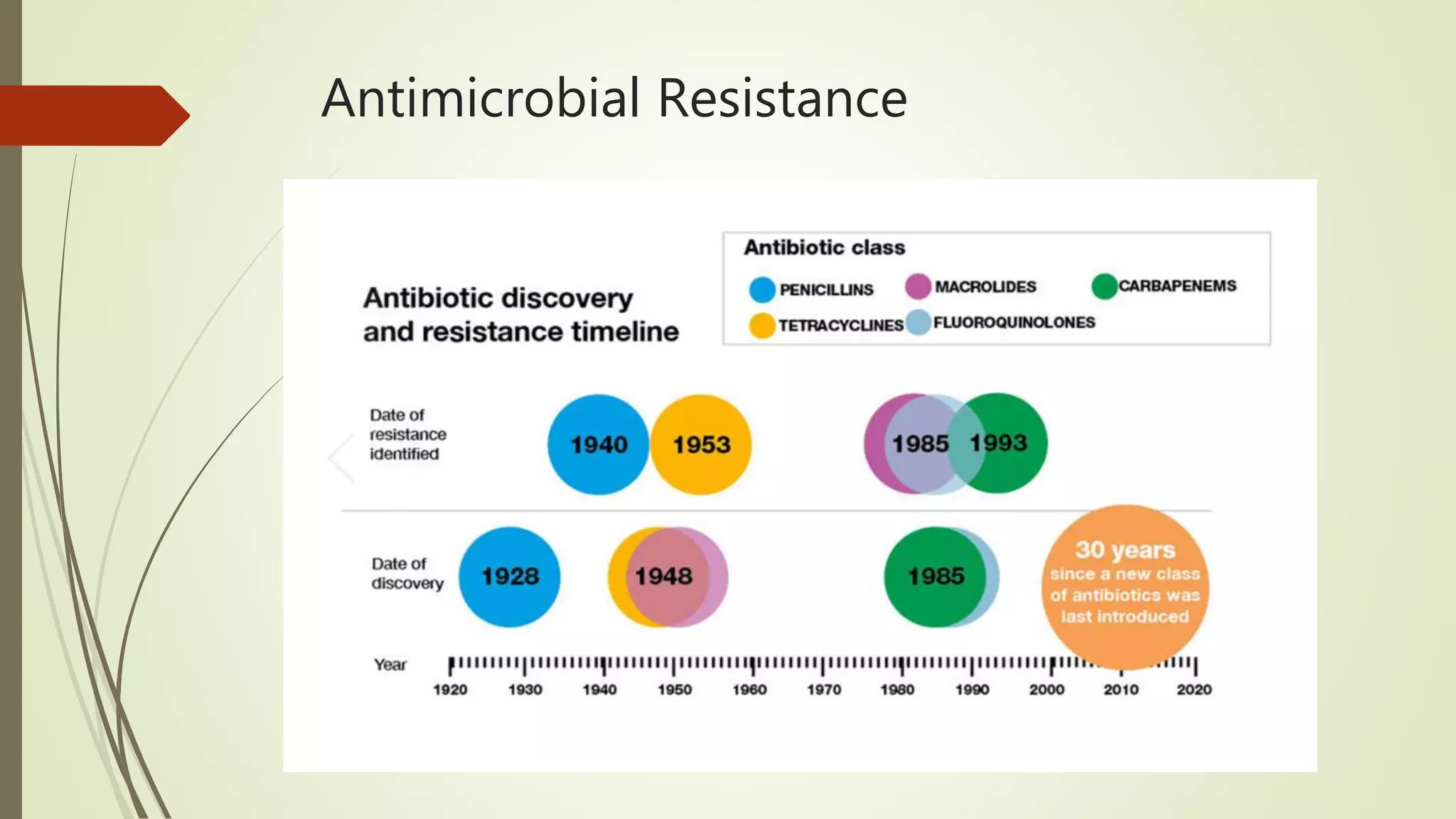
This document provides an overview of antimicrobial agents and antibiotics. It discusses the germ theory of disease, the timeline of antibiotic discovery, and classifications of antimicrobial agents. It describes different types of antimicrobial therapy including prophylaxis, empirical, and definitive therapy. Key concepts covered include bacteriostatic vs bactericidal agents, minimum inhibitory concentration, and principles of antibiotic dosing. The document also addresses factors influencing antimicrobial choice, problems with antimicrobial use including resistance, and Schedule HX regulations in India.