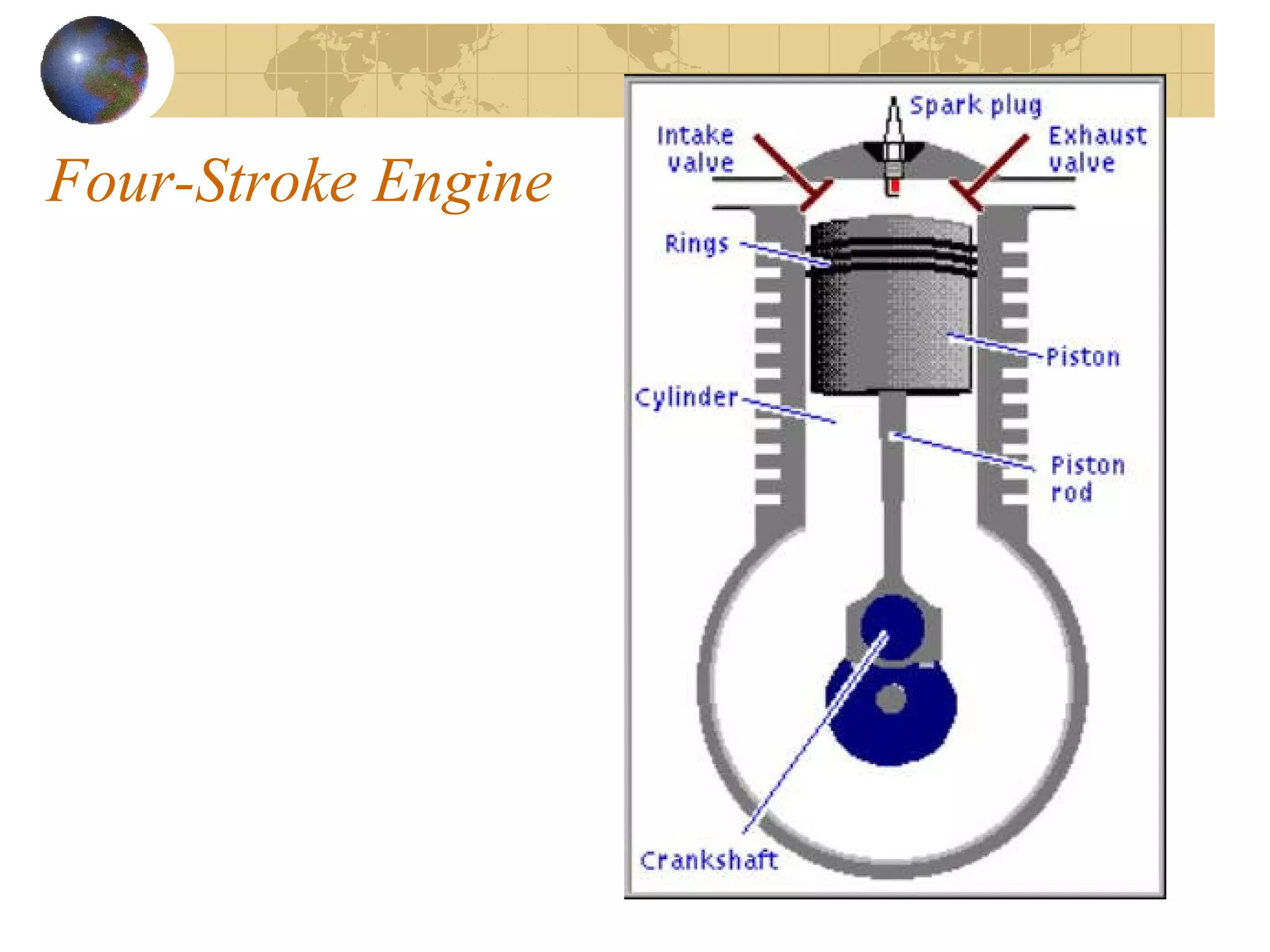
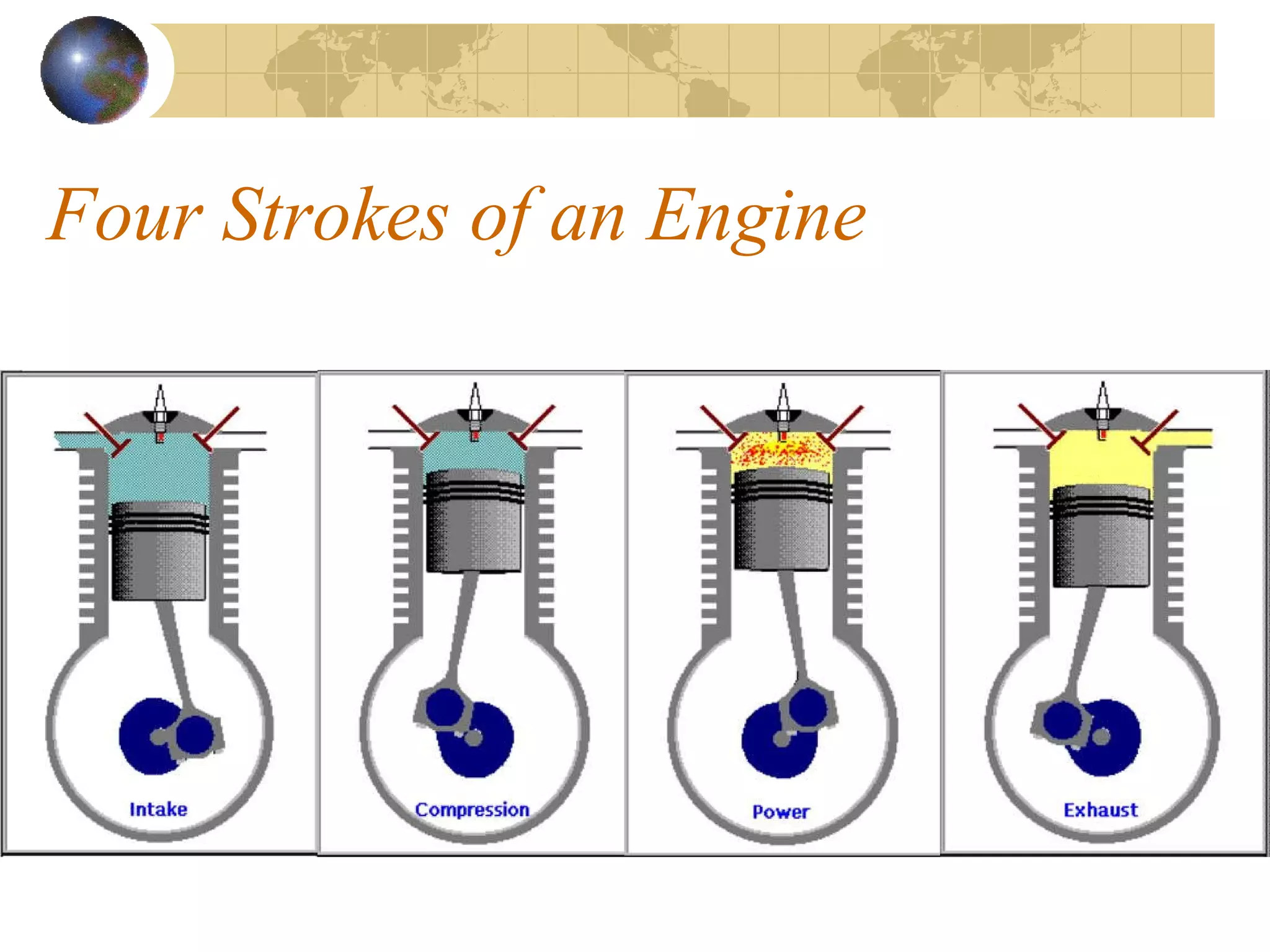
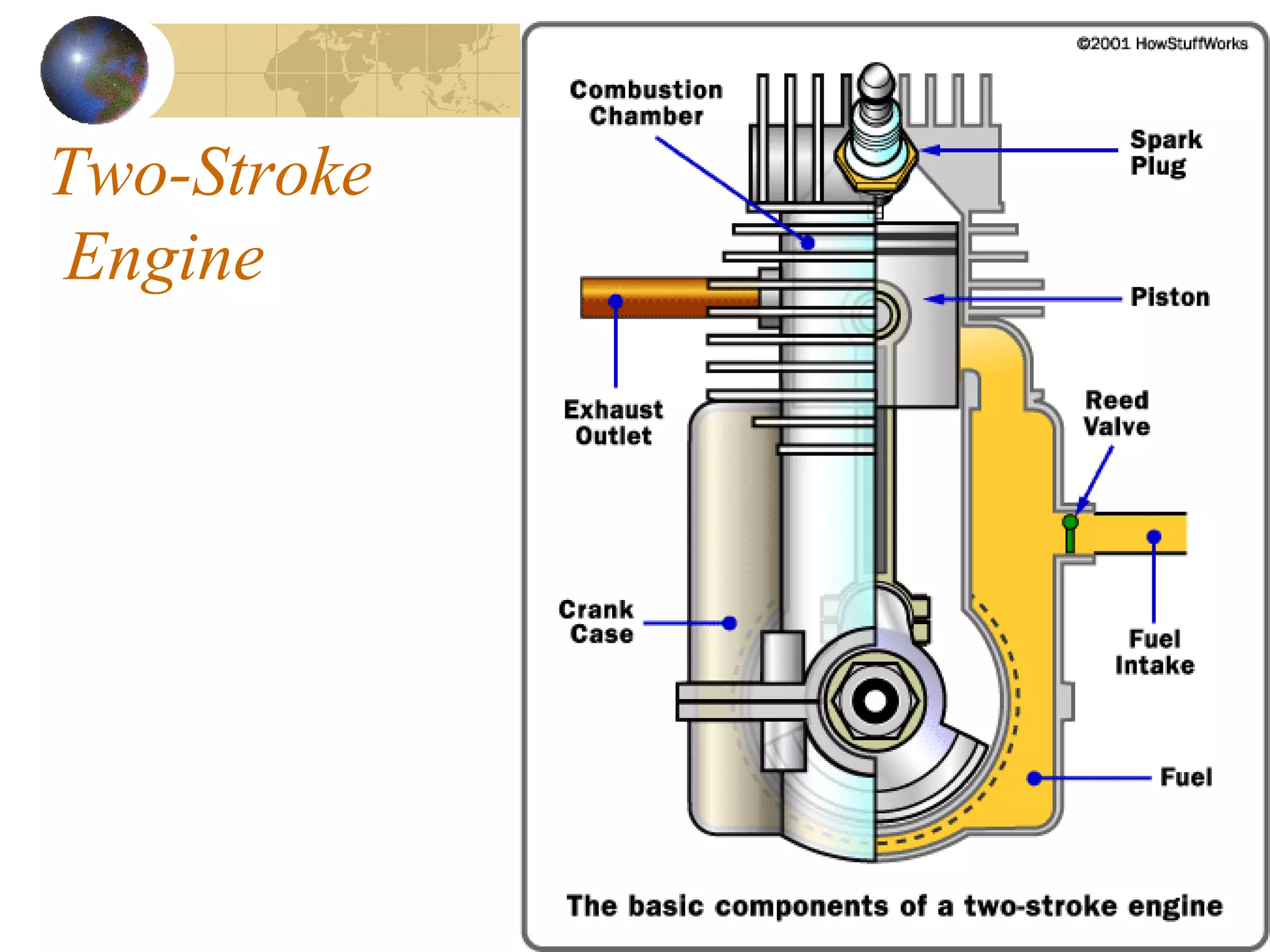
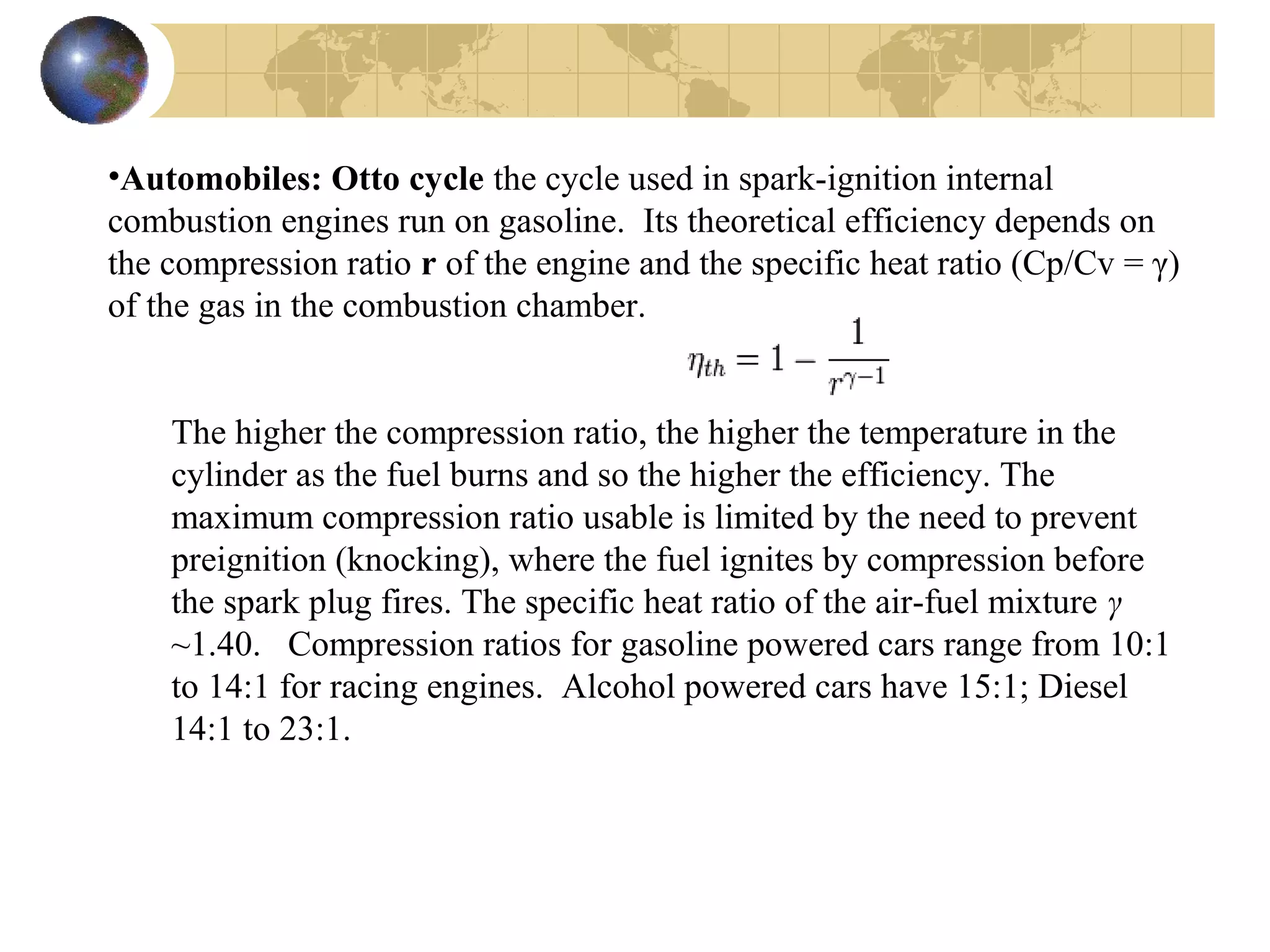
1. The document discusses internal combustion engines and the formation of gaseous pollutants and photochemical smog.
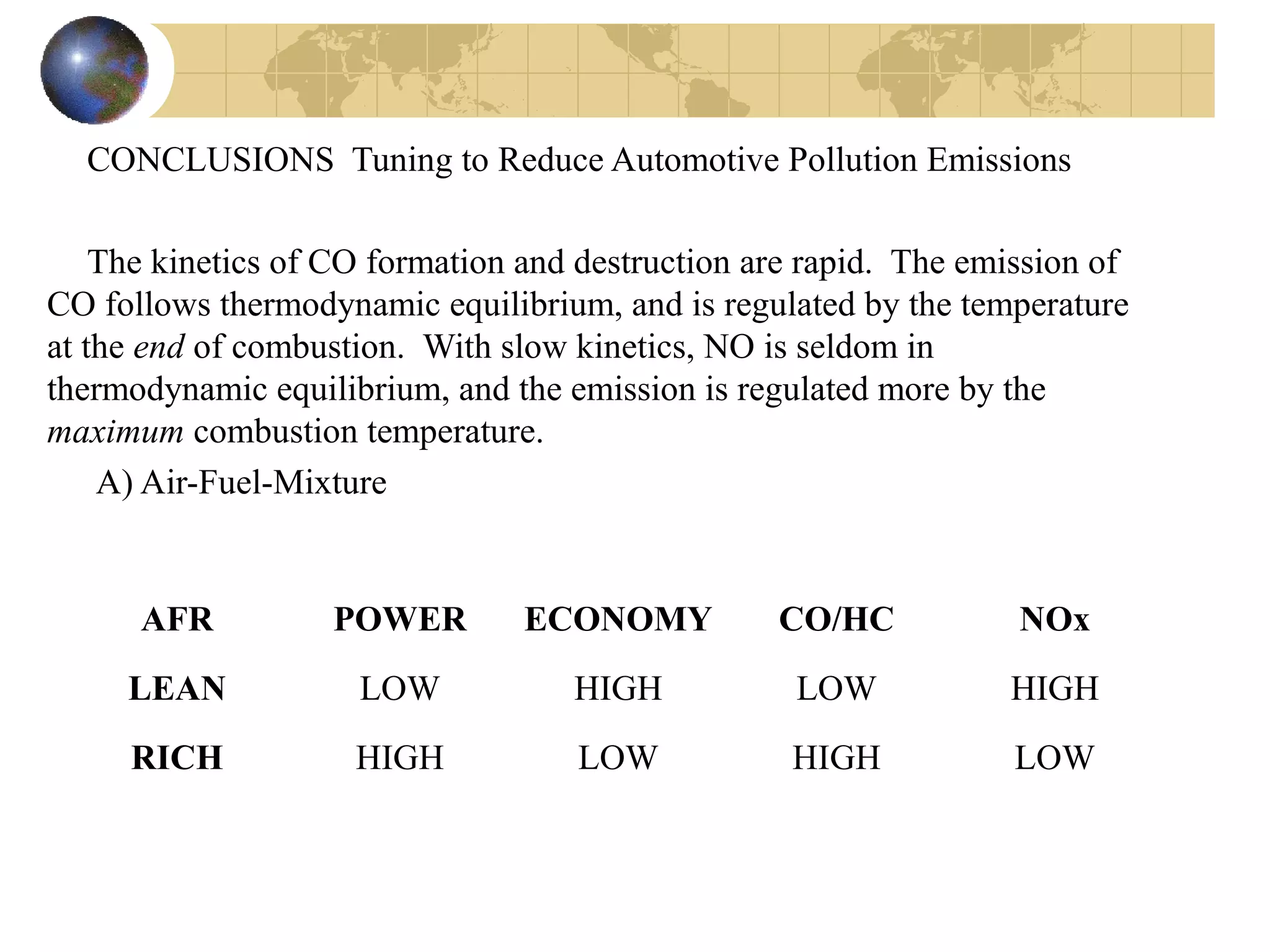
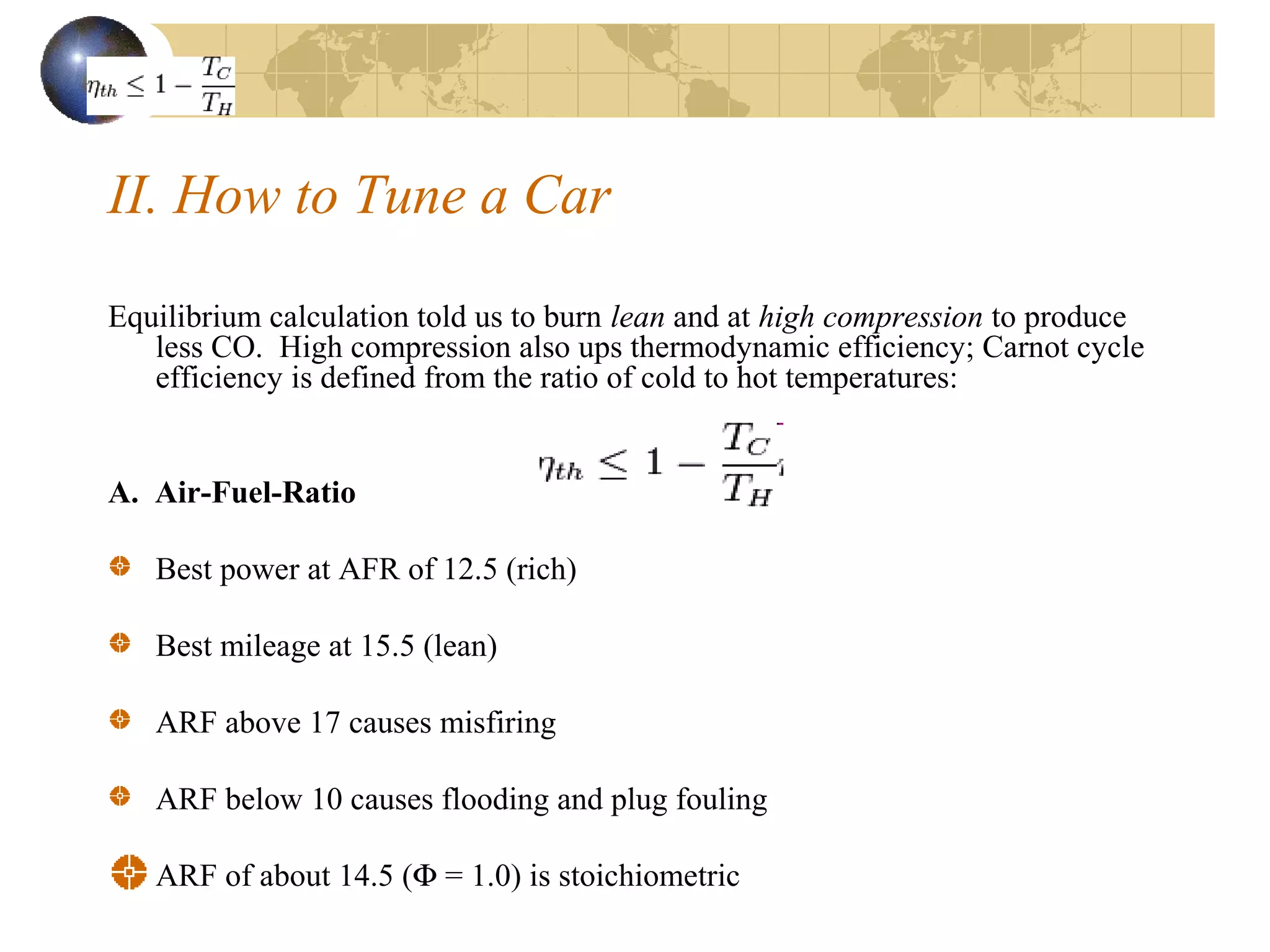
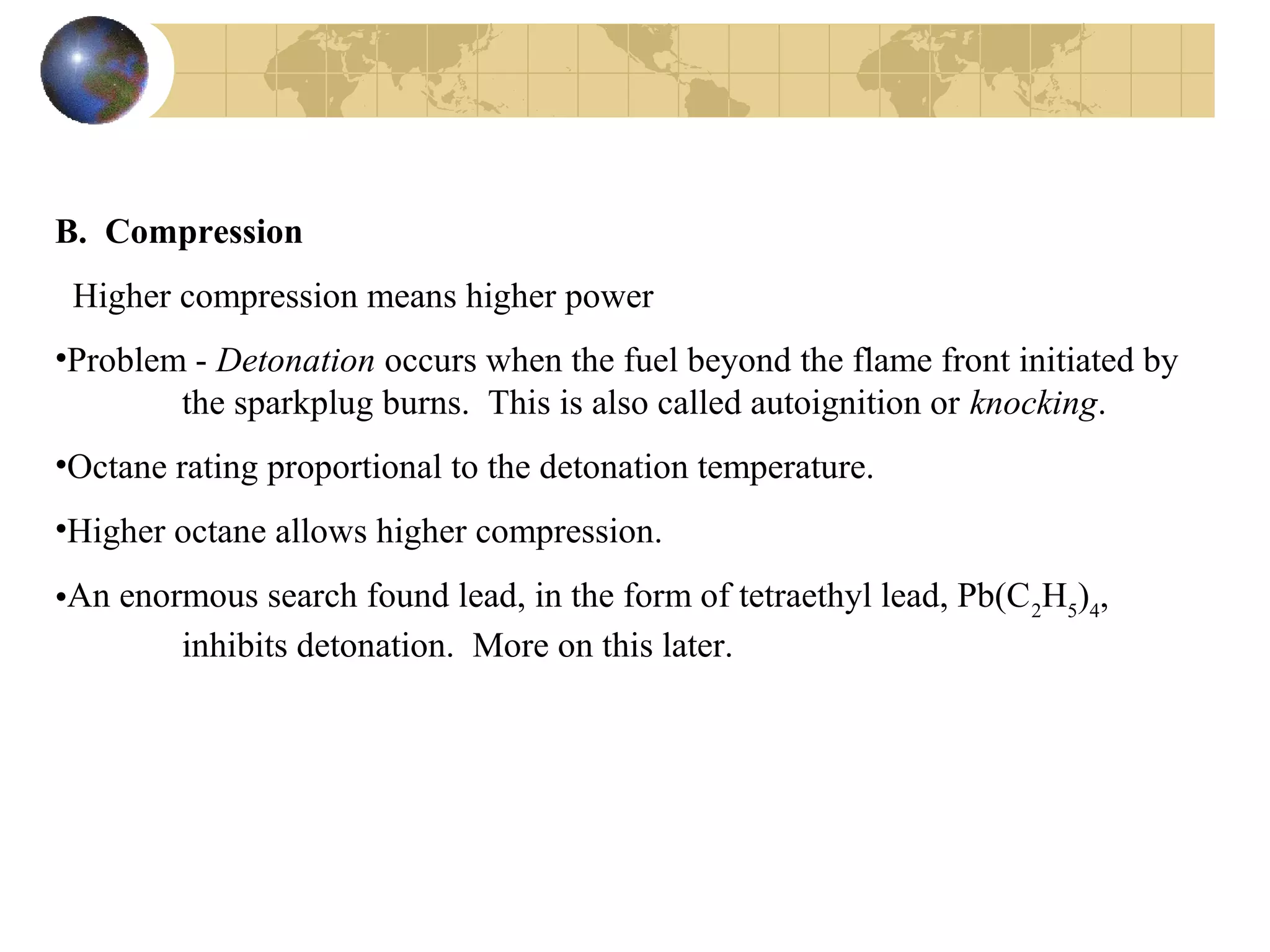
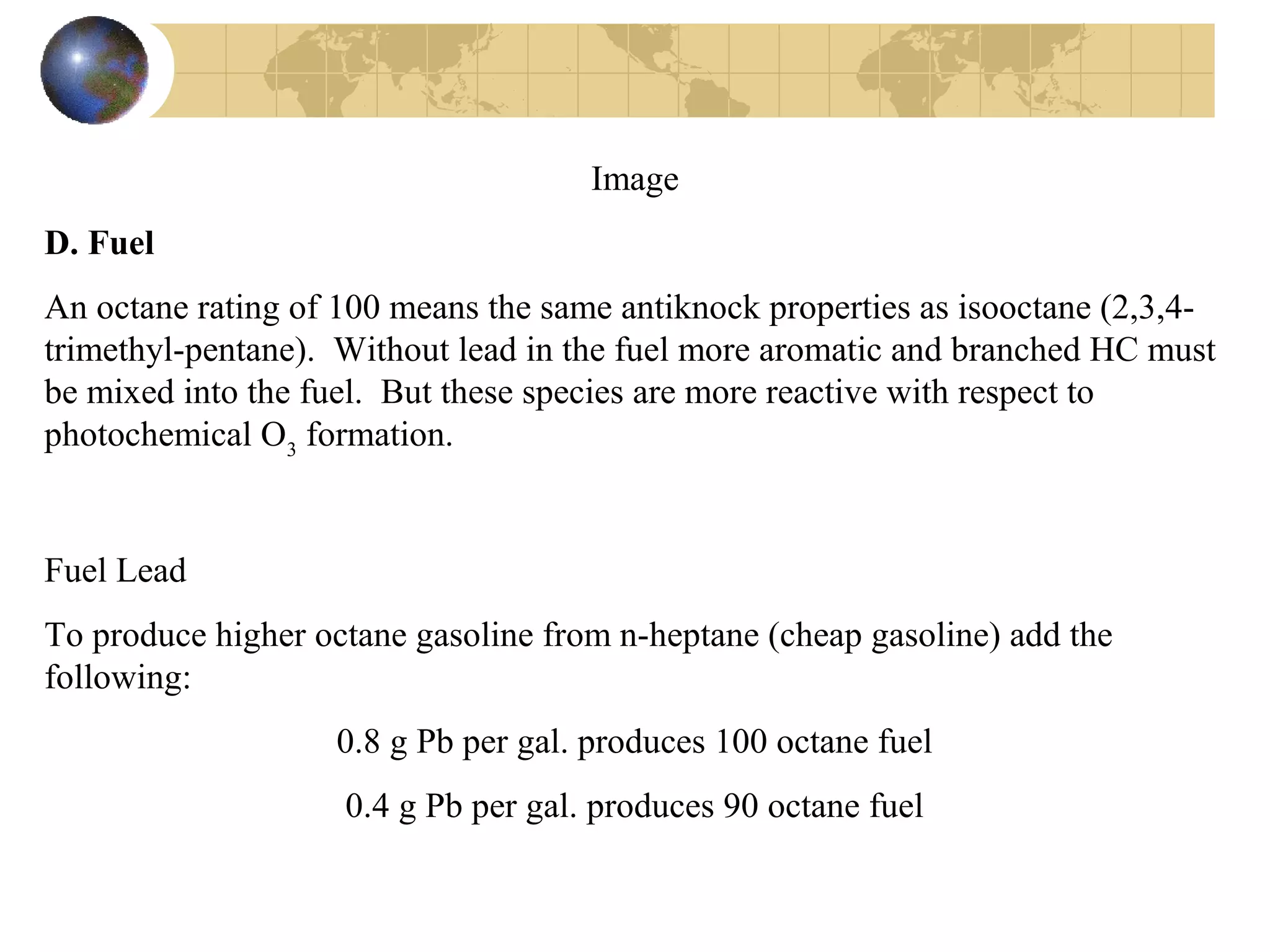
2. It describes how tuning factors like air-fuel ratio, compression, timing, and exhaust gas recycling can impact emissions of pollutants like carbon monoxide, hydrocarbons, and nitrogen oxides from automobile engines.
3. The formation of nitrogen oxides is explained through the Zeldovich mechanism and equations are provided for the rate of nitric oxide formation over time as exhaust gases cool.





![b) Carbon Monoxide
CO2
= CO + 1/2 O2
Keq
= e(-∆G/RT)
The process becomes kinetically limited as expansion occurs. The
formation of CO is quick, but the removal is slower, especially at
temperatures below about 1000 K. Thus the [CO] is close to the [CO]
calculated by the above equilibrium method based on the temperature of
the exhaust gases at the end of expansion.
Image](https://image.slidesharecdn.com/637internalcomb-150720195727-lva1-app6891/75/Internal-combustion-engine-22-2048.jpg)







![The Role of Internal Combustion in gaseous
pollution and Photochemical Smog
Formation
The Automobile
Seinfeld Chapt. 3
Wark and Warner Chapt. 10
4. Exhaust Emissions
c) Nitric Oxide, NO
The formation of NO is controlled by kinetics, not thermodynamic
equilibrium. High temperatures favor the formation of NO, and as the
exhaust gases cool the NO is frozen out because the reformation of N2
and
O2
is slow. See Wark and Warner section 8.4. Our objective here is to
derive an expression for the rate at which [NO] approaches the
equilibrium concentration, [NO]eq .](https://image.slidesharecdn.com/637internalcomb-150720195727-lva1-app6891/75/Internal-combustion-engine-32-2048.jpg)

![We can represent the formation of NO as a two step process.
O + N2
↔ NO + N (1)
N + O2
↔ NO + O (2)
----------------------
N2
+ O2
↔ 2NO (NET)
d[NO]/dt = k1
[O][N2
] - k-1
[NO][N] + k2
[N][O2
] - k-2
[NO][O] (I)
We will assume that N is in steady state. This is not the same as assuming it
is in thermodynamic equilibrium.](https://image.slidesharecdn.com/637internalcomb-150720195727-lva1-app6891/75/Internal-combustion-engine-34-2048.jpg)
![Yakov Borisovich Zel'dovich
Awarded the Order of Lenin (1949)
d[N]/dt = k1
[O] [N2
] - k-1
[NO][N] - k2
[N][O2
] + k-2
[NO][O]
k1
[O] [N2
] + k-2
[NO][O]
[N]ss = --------------------------------
k-1
[NO] + k2
[O2
]
[O] { k1
[N2
] + k-2
[NO] }
[N]ss = --------------------------------- (II)
k-1
[NO] + k2
[O2
]](https://image.slidesharecdn.com/637internalcomb-150720195727-lva1-app6891/75/Internal-combustion-engine-35-2048.jpg)
![From I and II
2[O]k1
[N2
] - (k-1
k-2
[NO]2
/ k2
[O2
])
d[NO]/dt = ---------------------------------------------- (III)
1 + (k-1
[NO] / k-2
[O2
])
Where:
k1
= 1.3E-10 exp (-38000/T) cm3
s-1
k1
(2400 K) = 1.7E-17 cm3
s-1
k-1
= 3.4E-11 cm3
s-1
k-1(240K) = 3.4E -11 cm3
s-1
k2
= 1.5E-11 exp(-3600/T) cm3
s-1
k2
(2400 K) = 3.3E-12 cm3
s-1
k-2 = 2.5E -15 T exp(-19500/T) cm3
s-1
k-2 (2400 K) = 2E-15 cm3
s-1](https://image.slidesharecdn.com/637internalcomb-150720195727-lva1-app6891/75/Internal-combustion-engine-36-2048.jpg)
![K1
K2
= (k1
/k-1
)(k2
/k-2
) = (PNO
)2
/{ PN2
PO2
} (IV)
2k1
[O][N2
] {1 - ([NO]2
/ Keq [N2
] [O2
])}
d[NO]/dt = -------------------------------------------------
1 + (k1
[NO] / k2
[O2
])](https://image.slidesharecdn.com/637internalcomb-150720195727-lva1-app6891/75/Internal-combustion-engine-38-2048.jpg)
![For a given temperature, Equation IV can be integrated to yield an
expression for the concentration of NO as a function of time, but this is a
tedious process. See Wark and Warner, p. 384. The result is:
[NO]t = [NO]eq ( 1 - (exp(-Mt))1/2
)
Where [NO]eq is the equilibrium concentration of NO and
M = 5.7E15 T -1
P1/2
exp(-58400/T) s-1
Note that M is a strong function of temperature, but not pressure. We have
assumed that Reactions 1 and 2 control, that the temperature is constant
throughout the process, and that N2
and O2
are present at a ratio of 40:1. The
actual process is very complicated because the temperature does not remain
constant.](https://image.slidesharecdn.com/637internalcomb-150720195727-lva1-app6891/75/Internal-combustion-engine-39-2048.jpg)