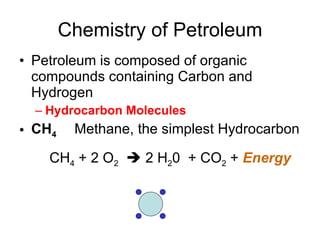
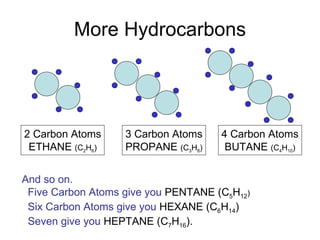
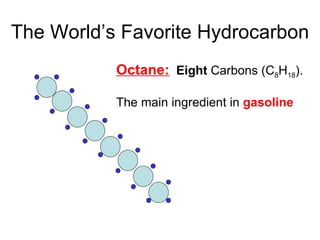
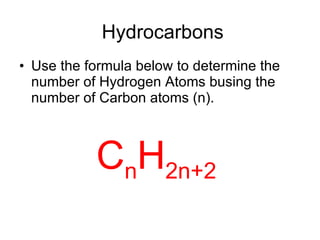
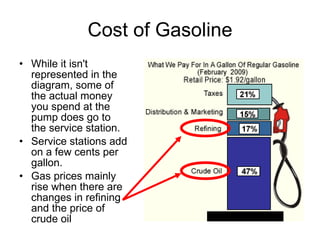
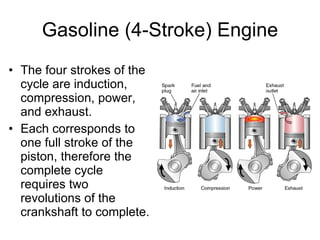
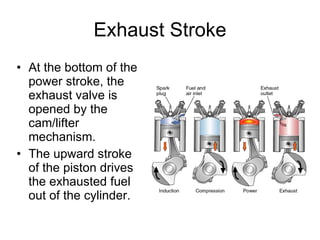
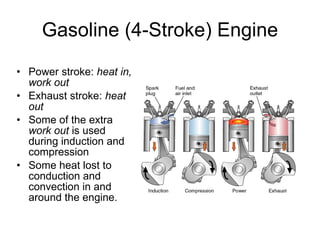
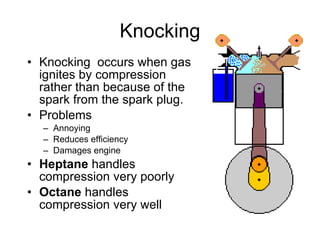
The document discusses the chemistry of petroleum and gasoline. Petroleum is composed of hydrocarbon molecules with varying numbers of carbon atoms. Crude oil is extracted from wells as a mixture of hydrocarbons and refined into useful products through fractional distillation and cracking/reforming processes. Gasoline is the portion of refined petroleum containing 8 carbon hydrocarbons that vaporize at low temperatures, making it a suitable fuel for combustion engines. Octane rating and additives like tetraethyl lead were historically used to prevent engine knocking, though lead posed health and environmental risks.