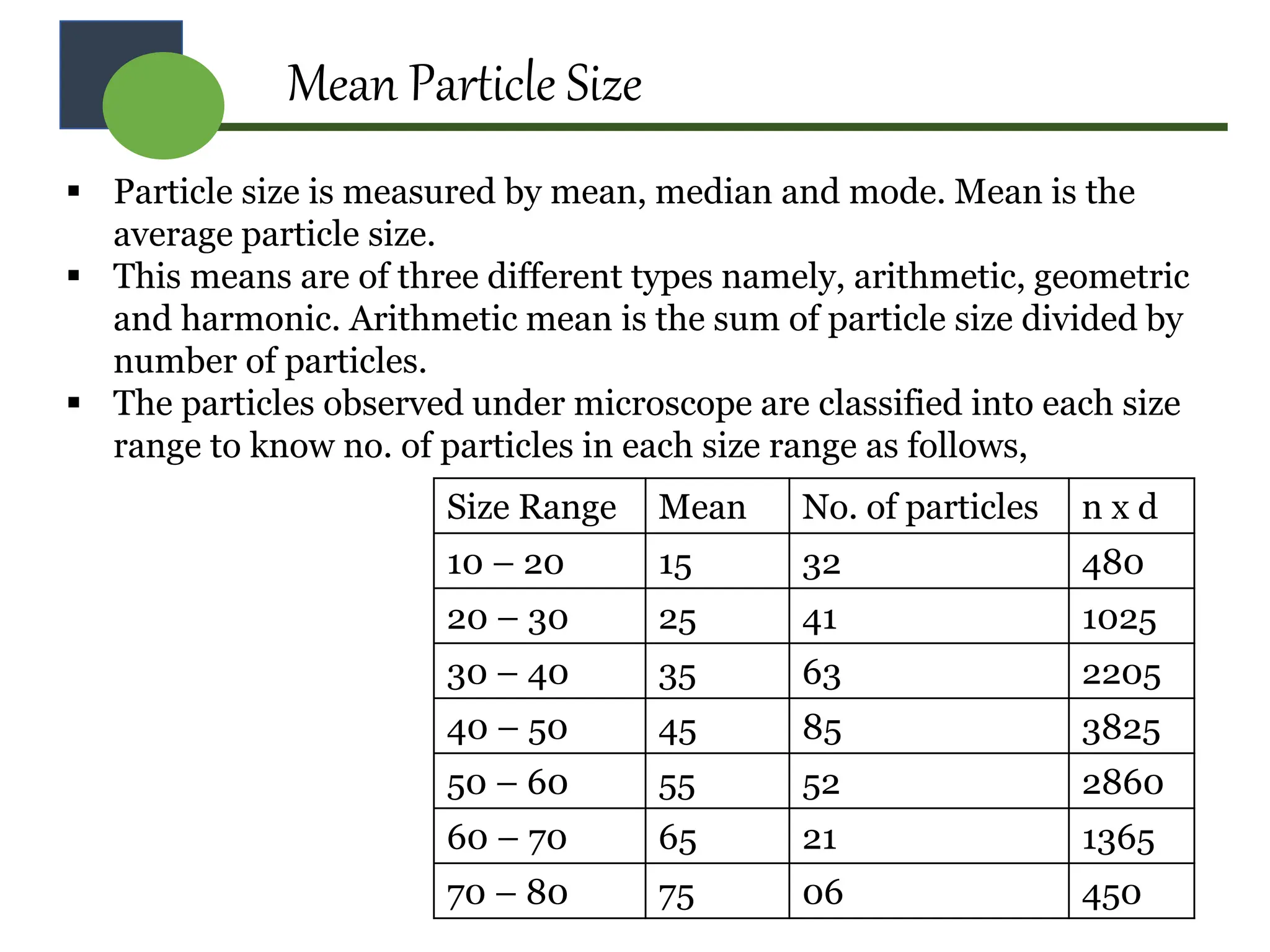
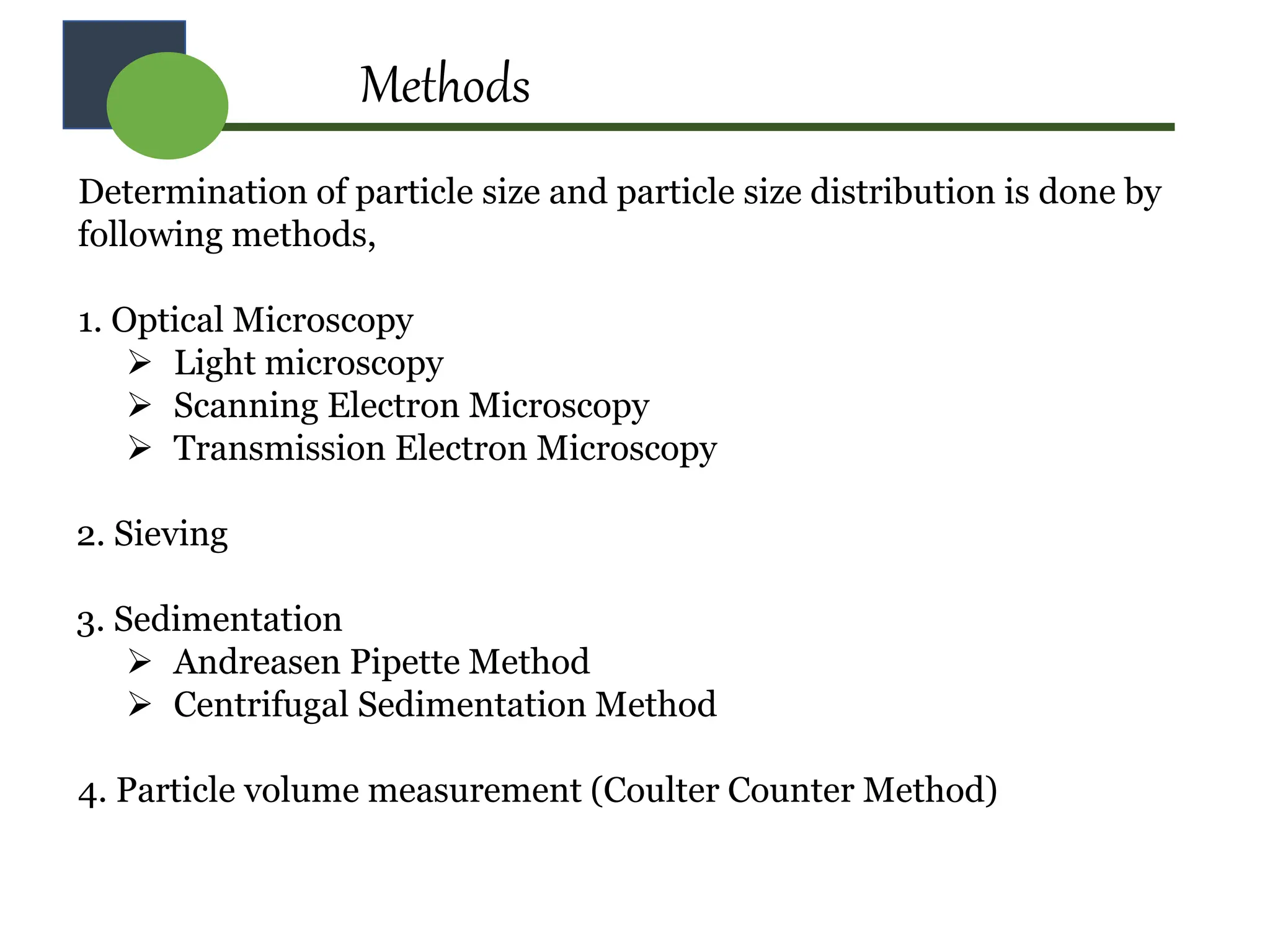
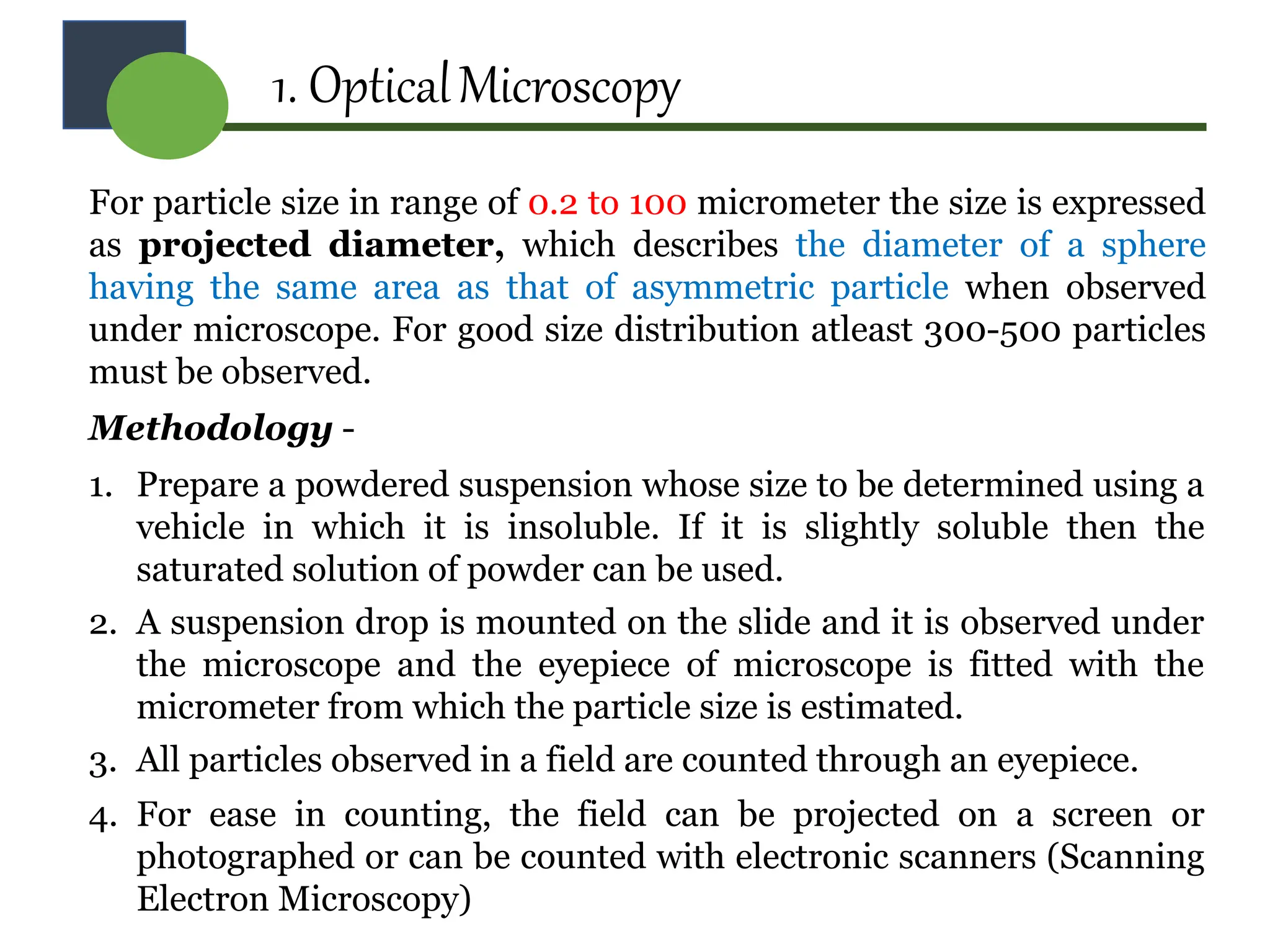
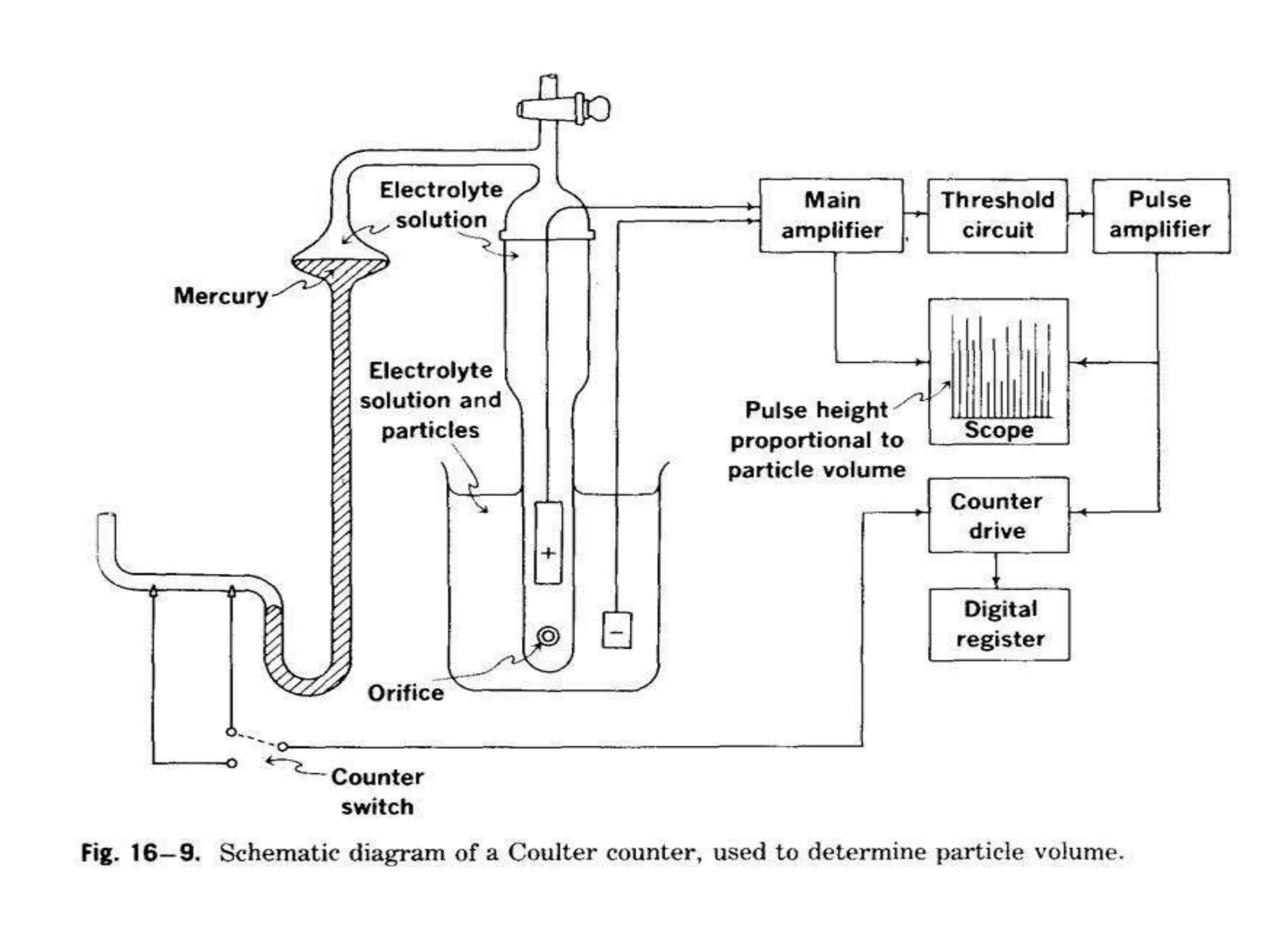
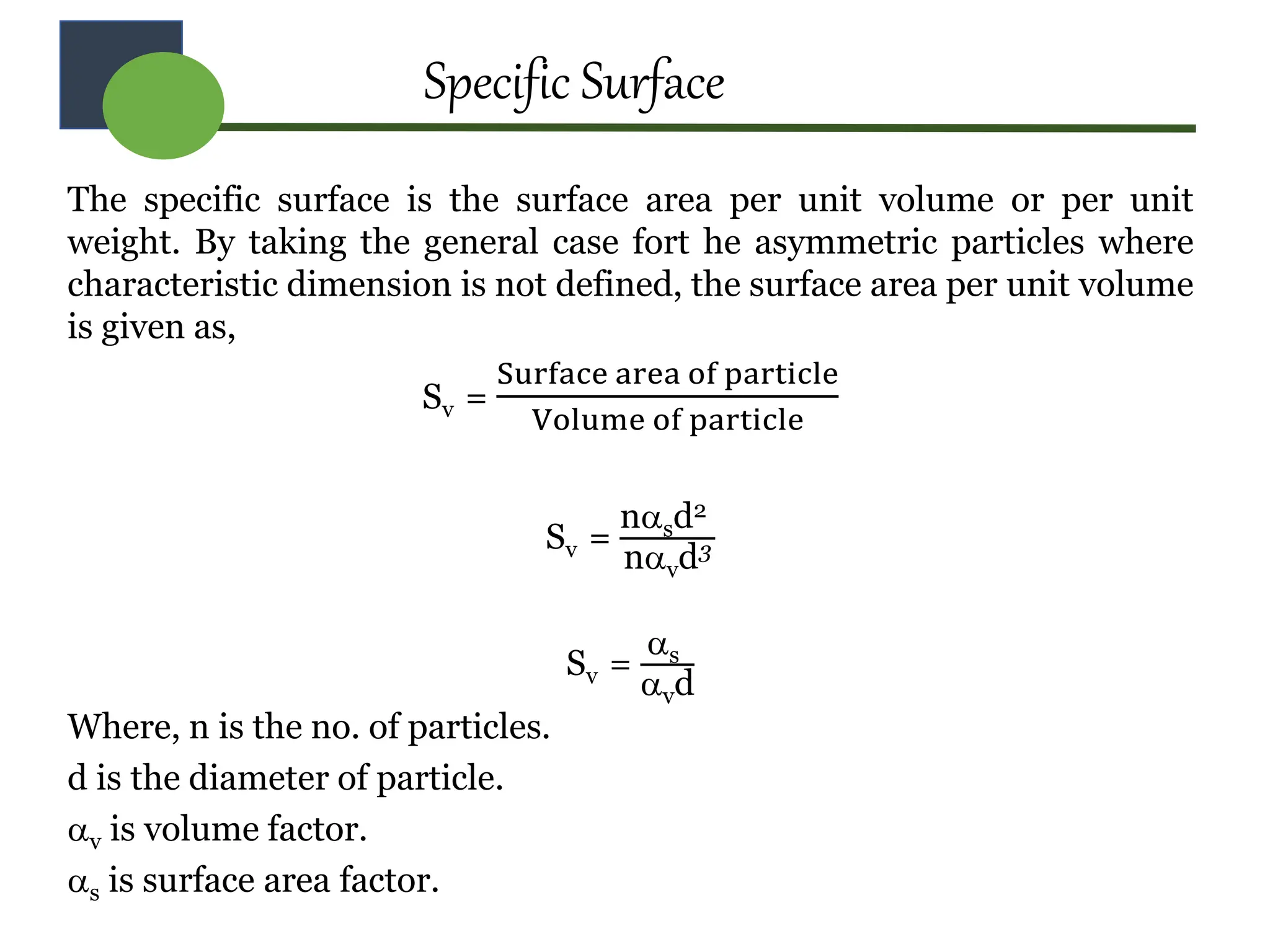
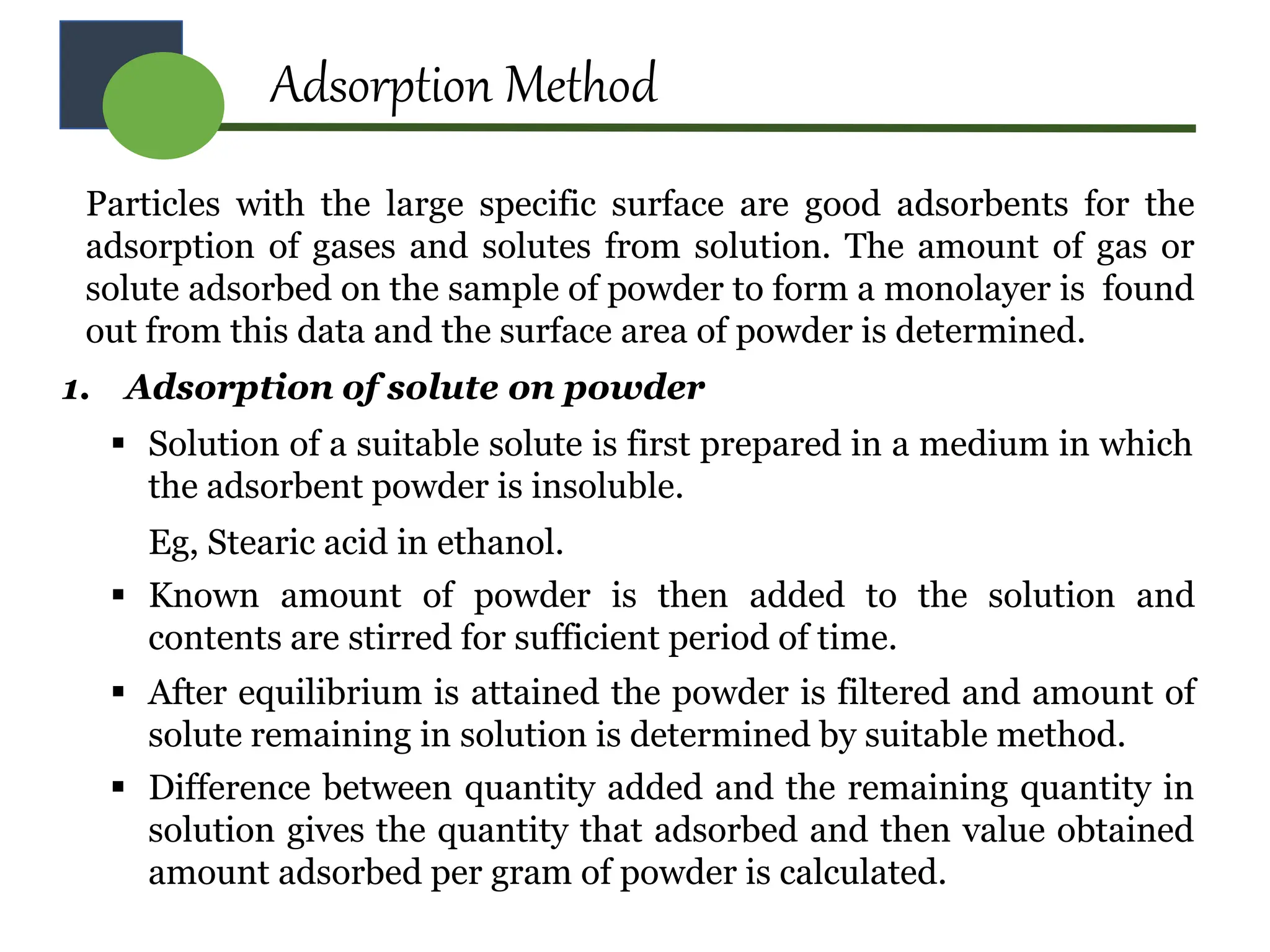
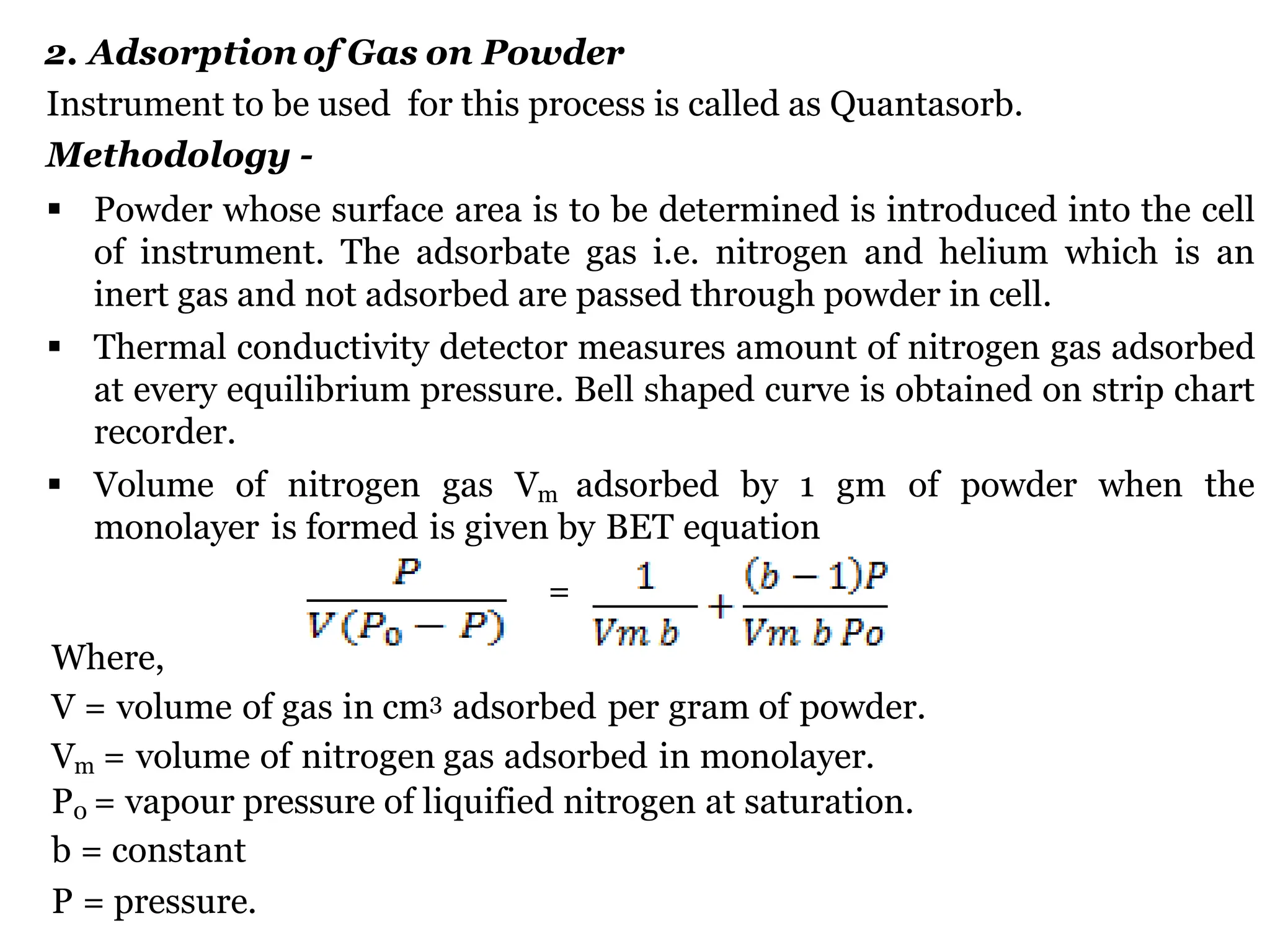
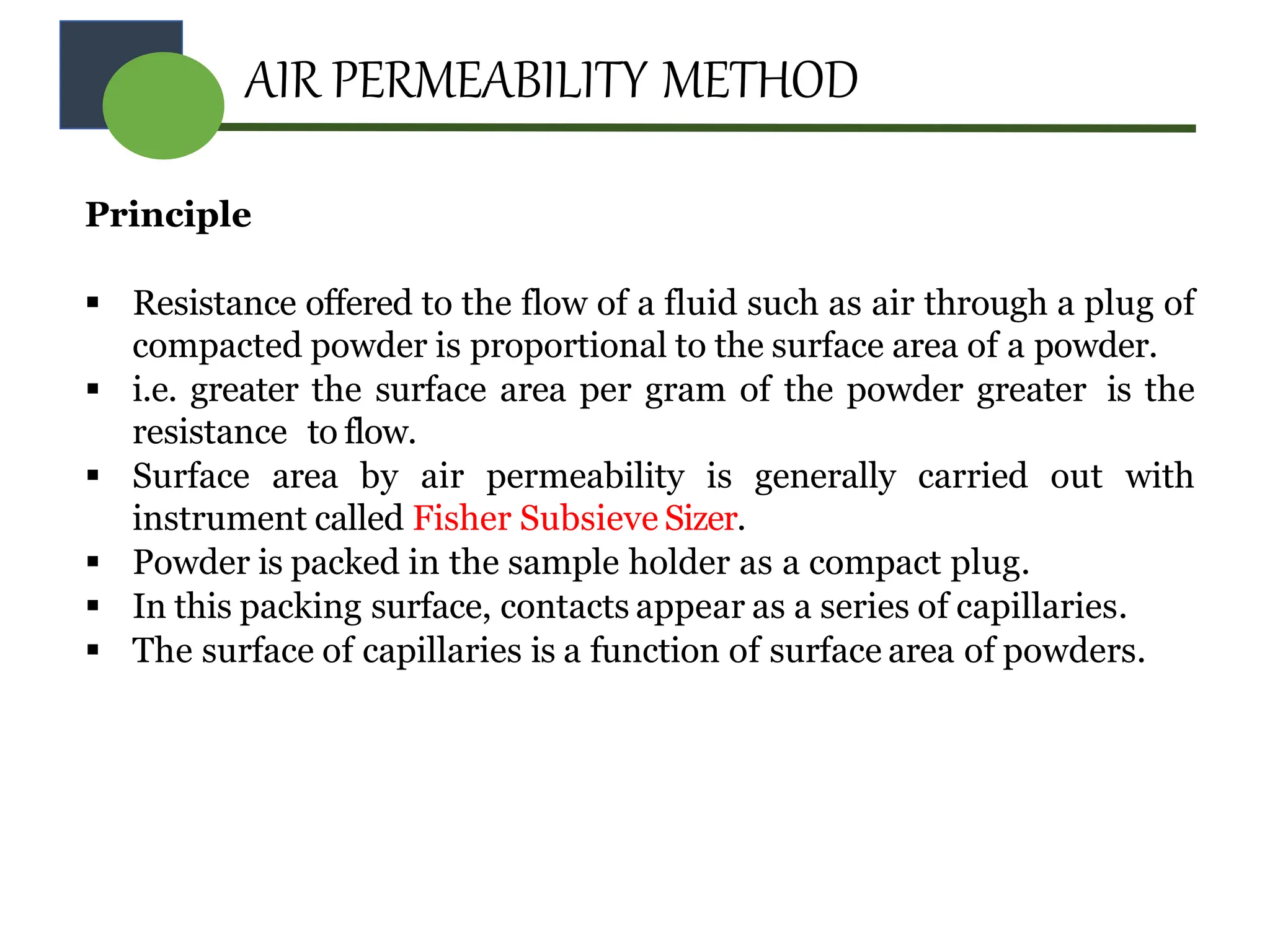
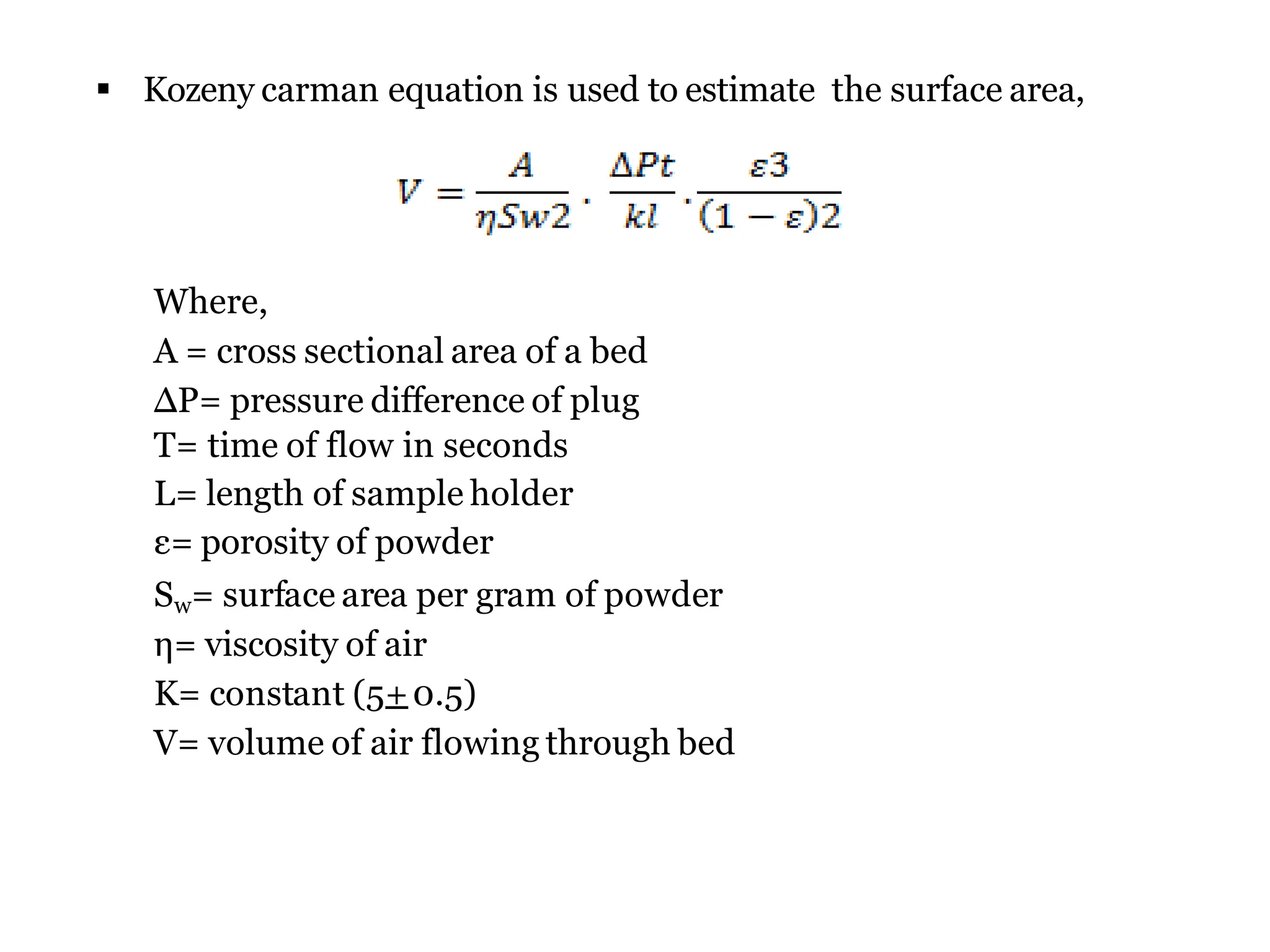
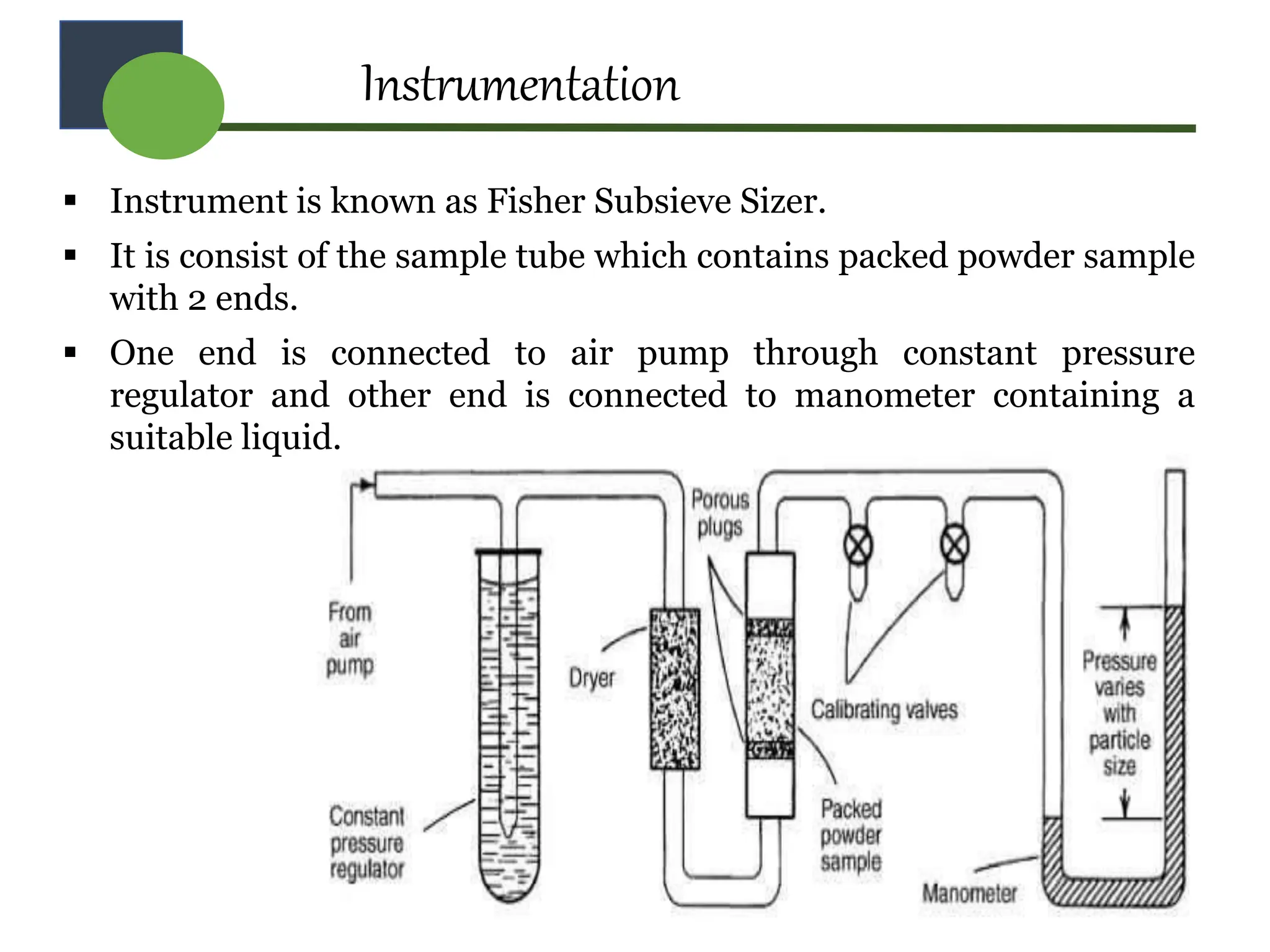
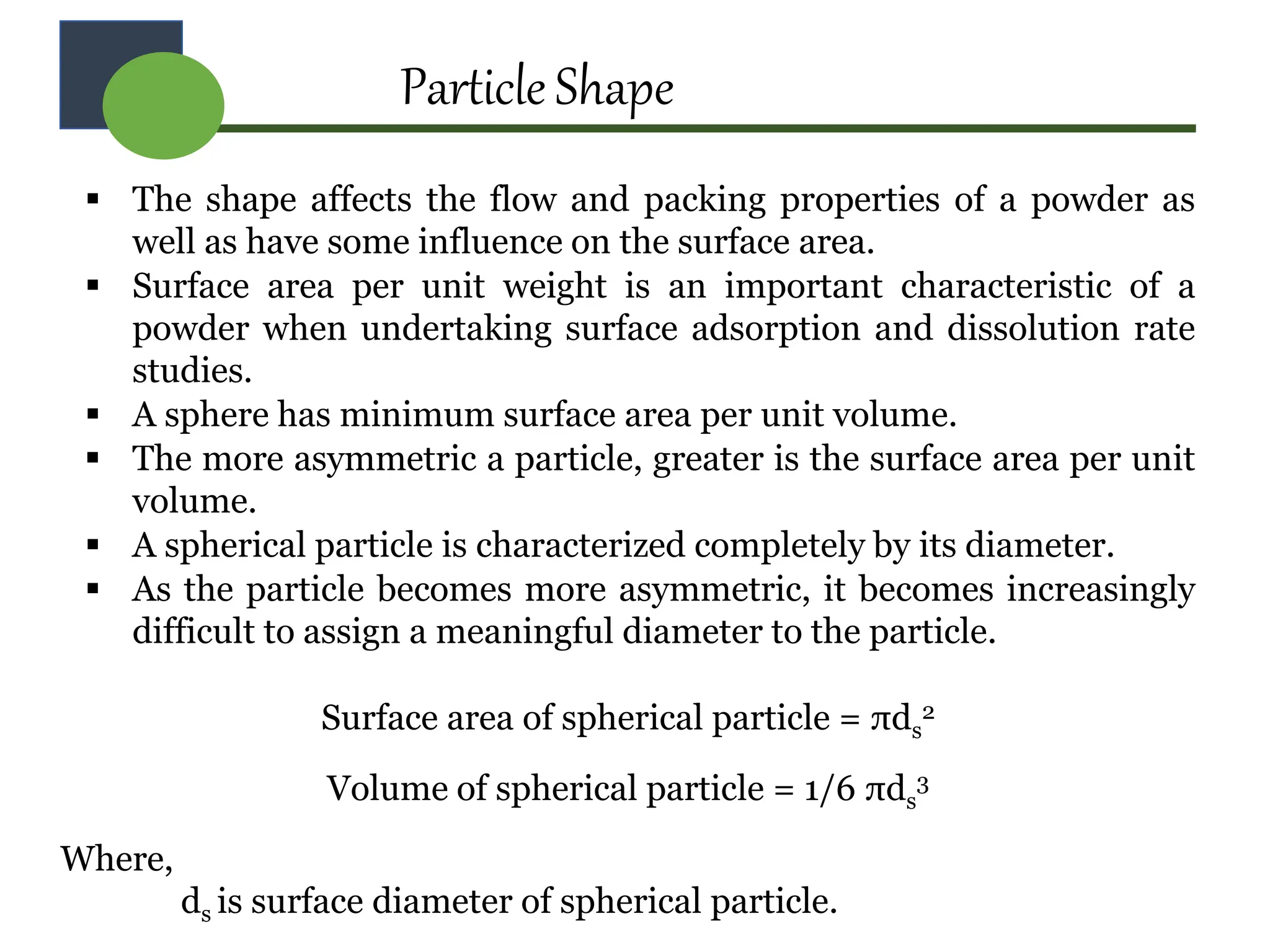
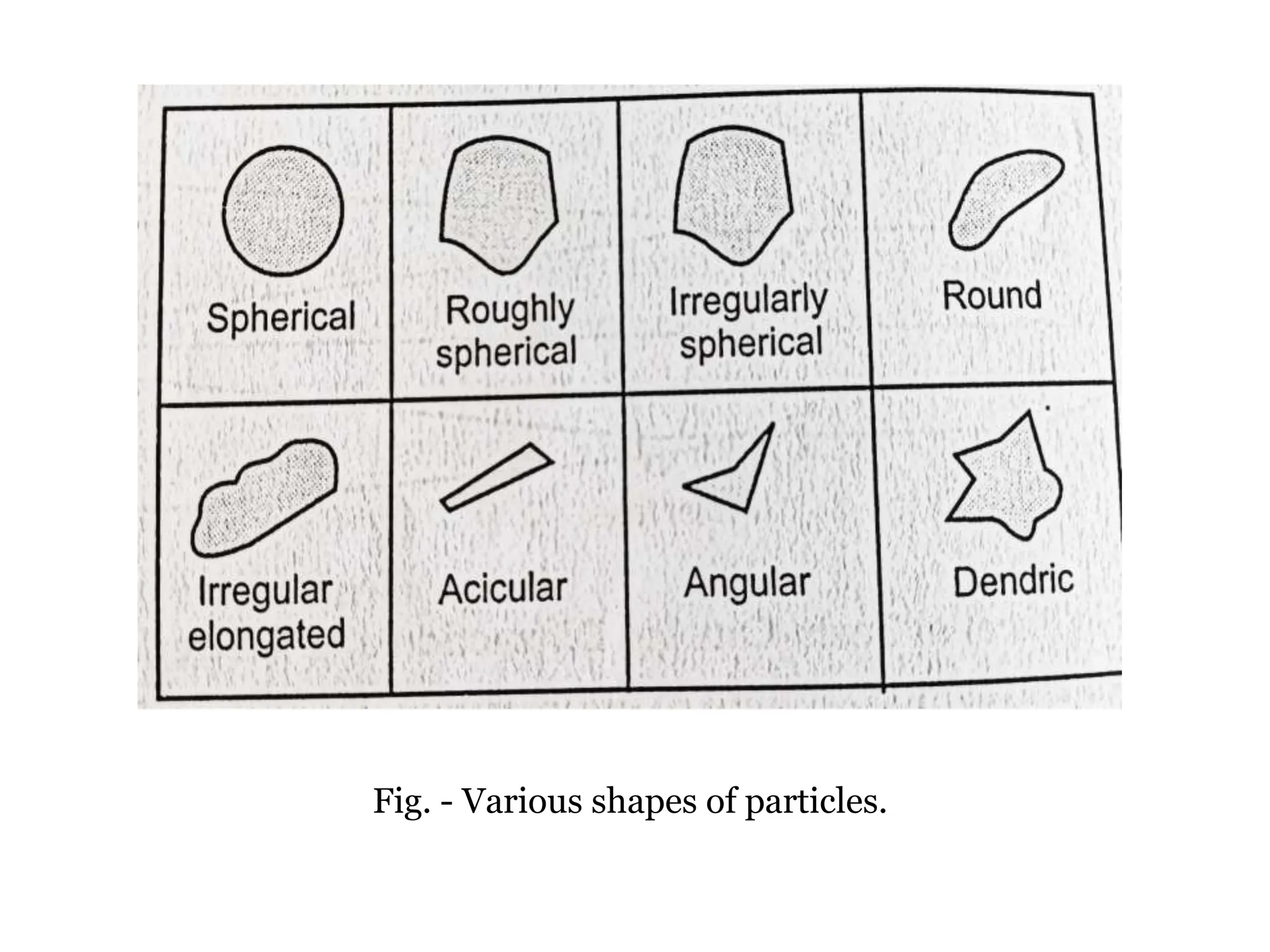
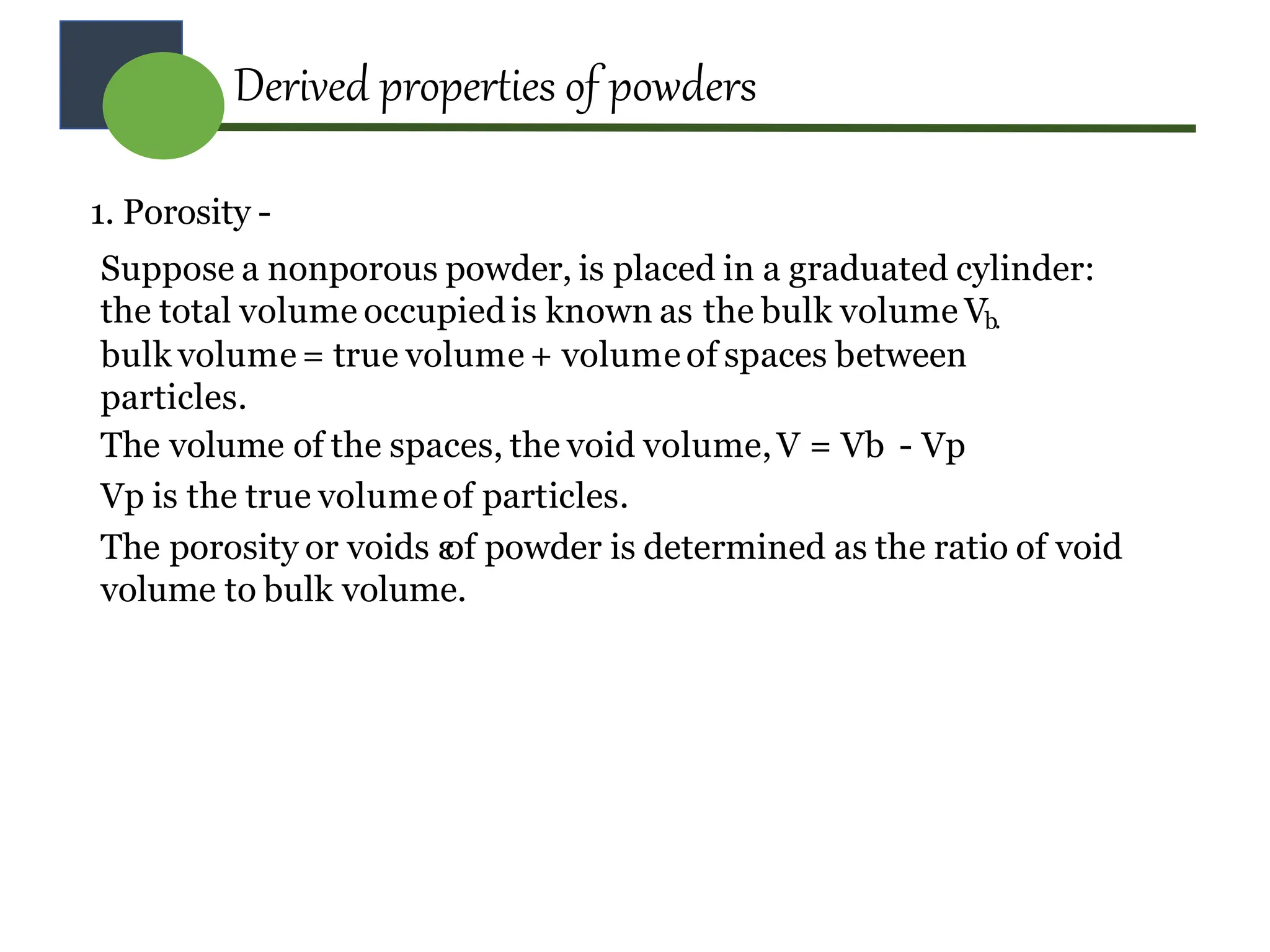
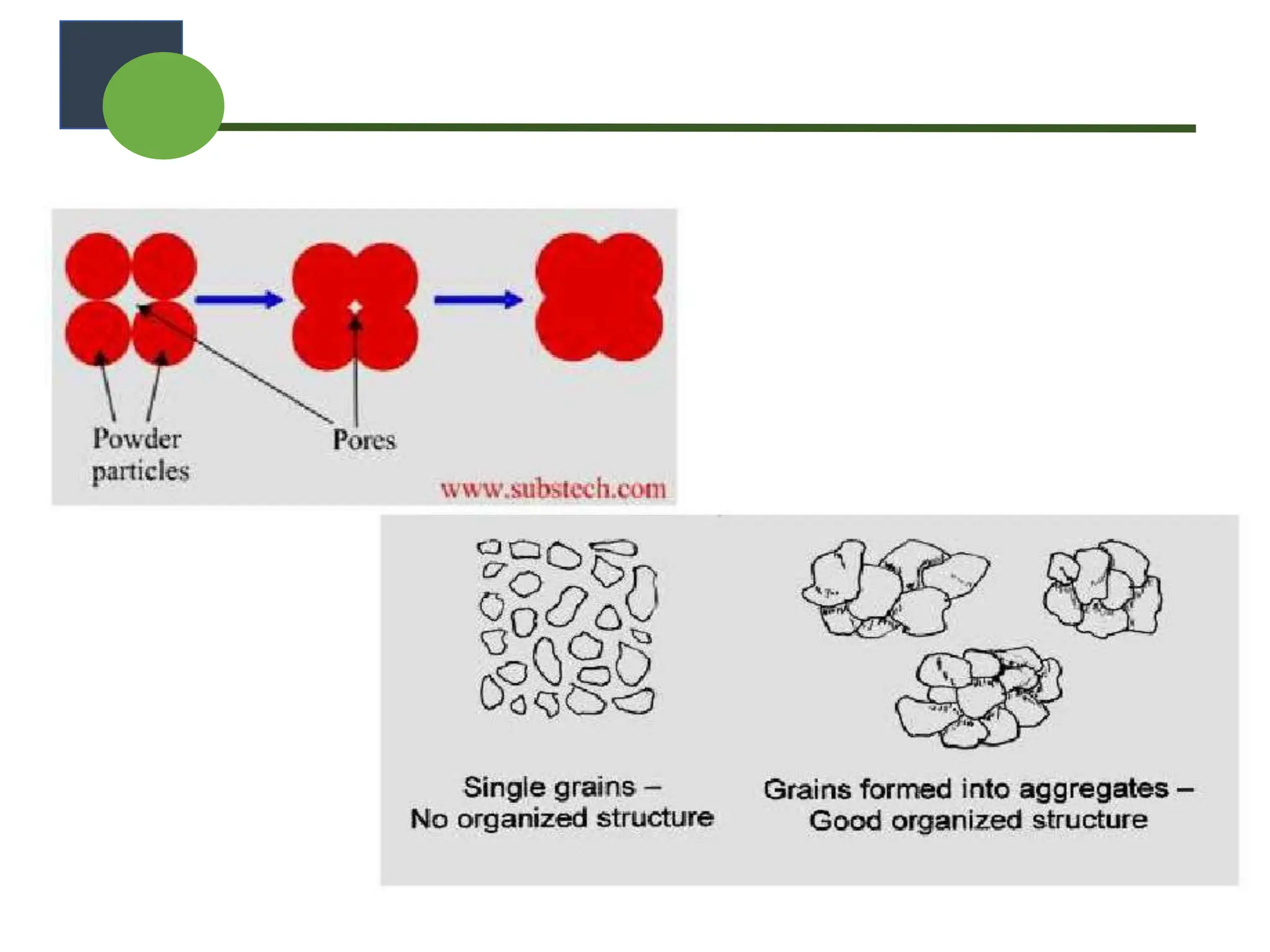
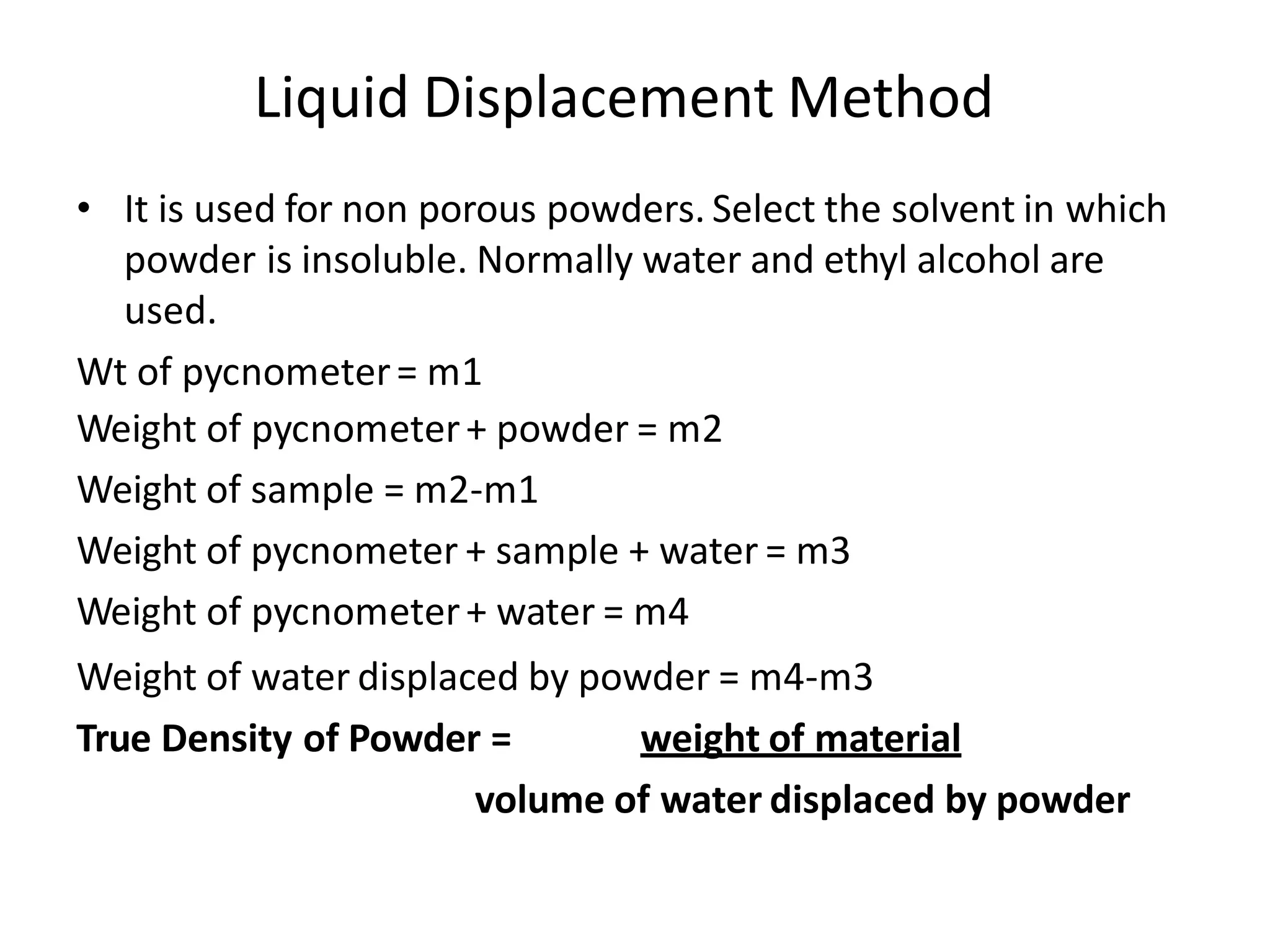
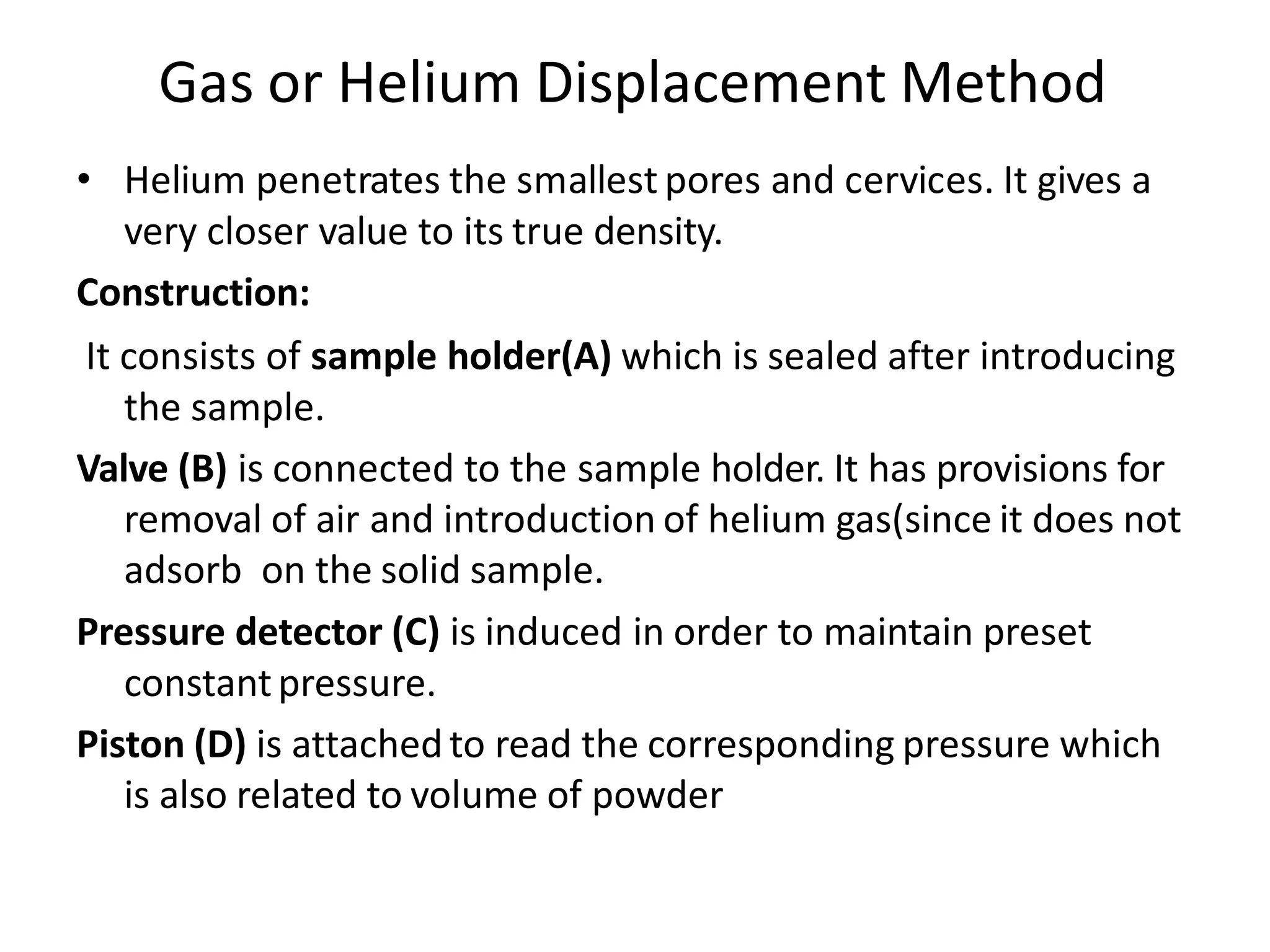
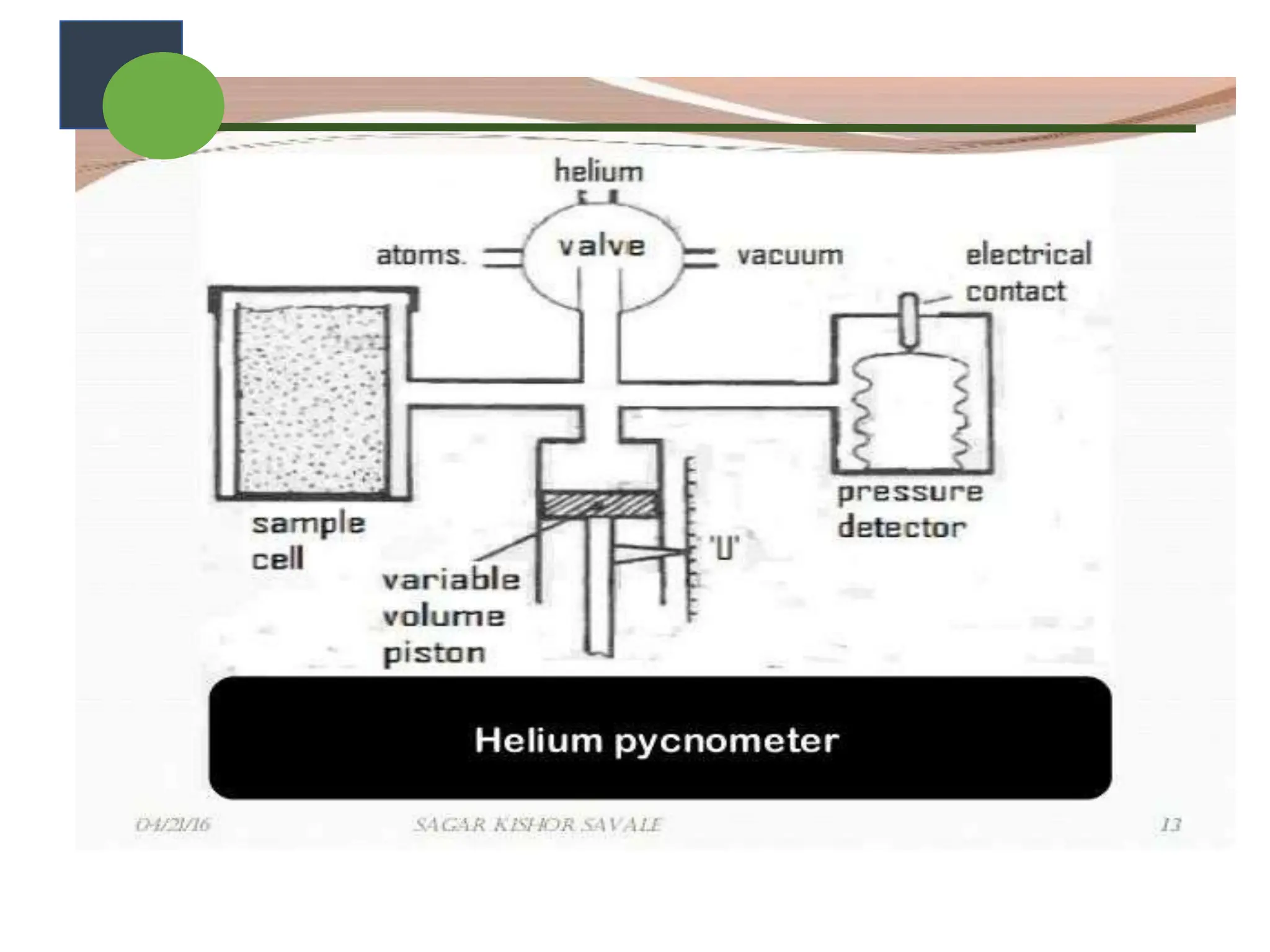
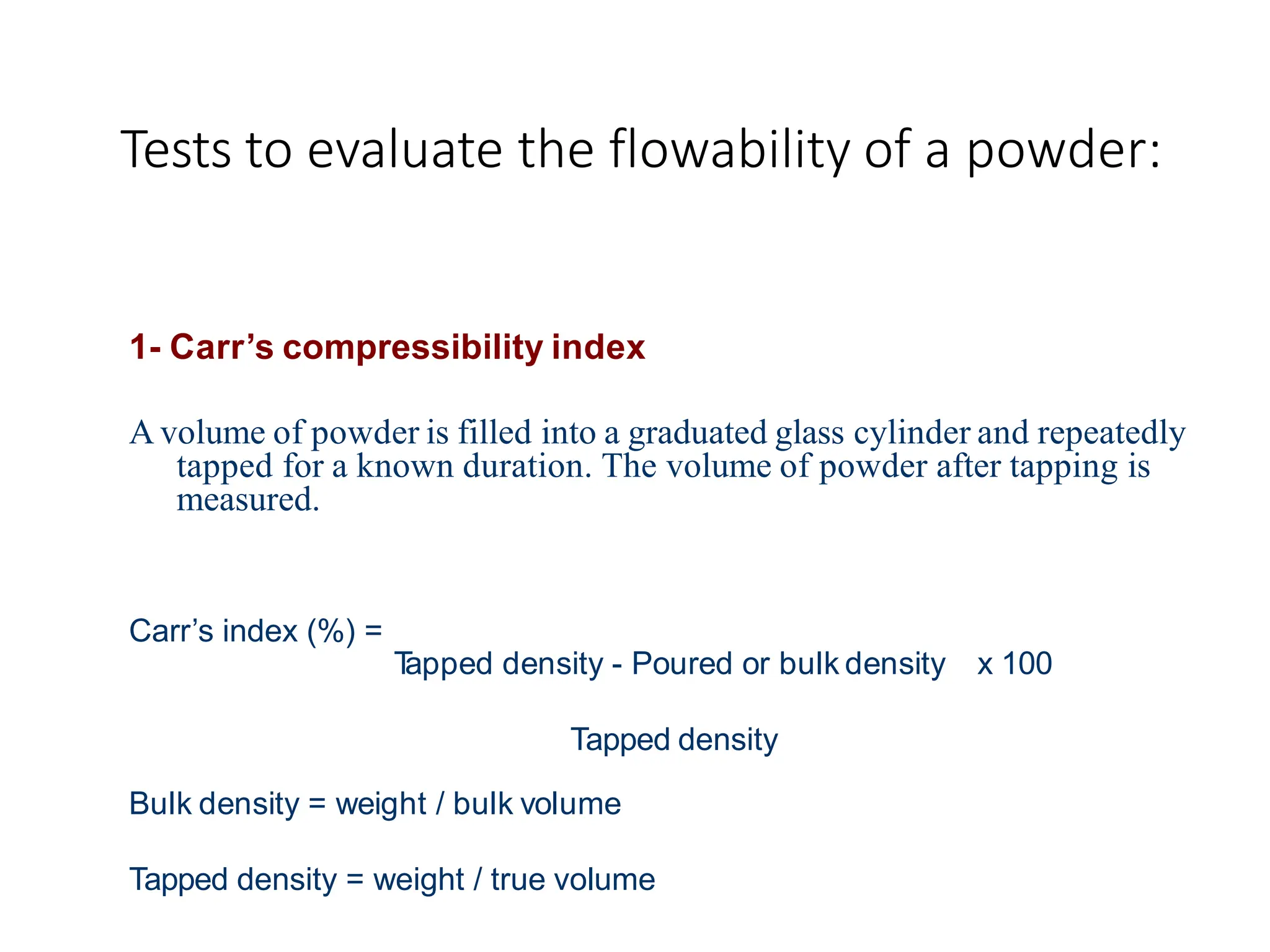
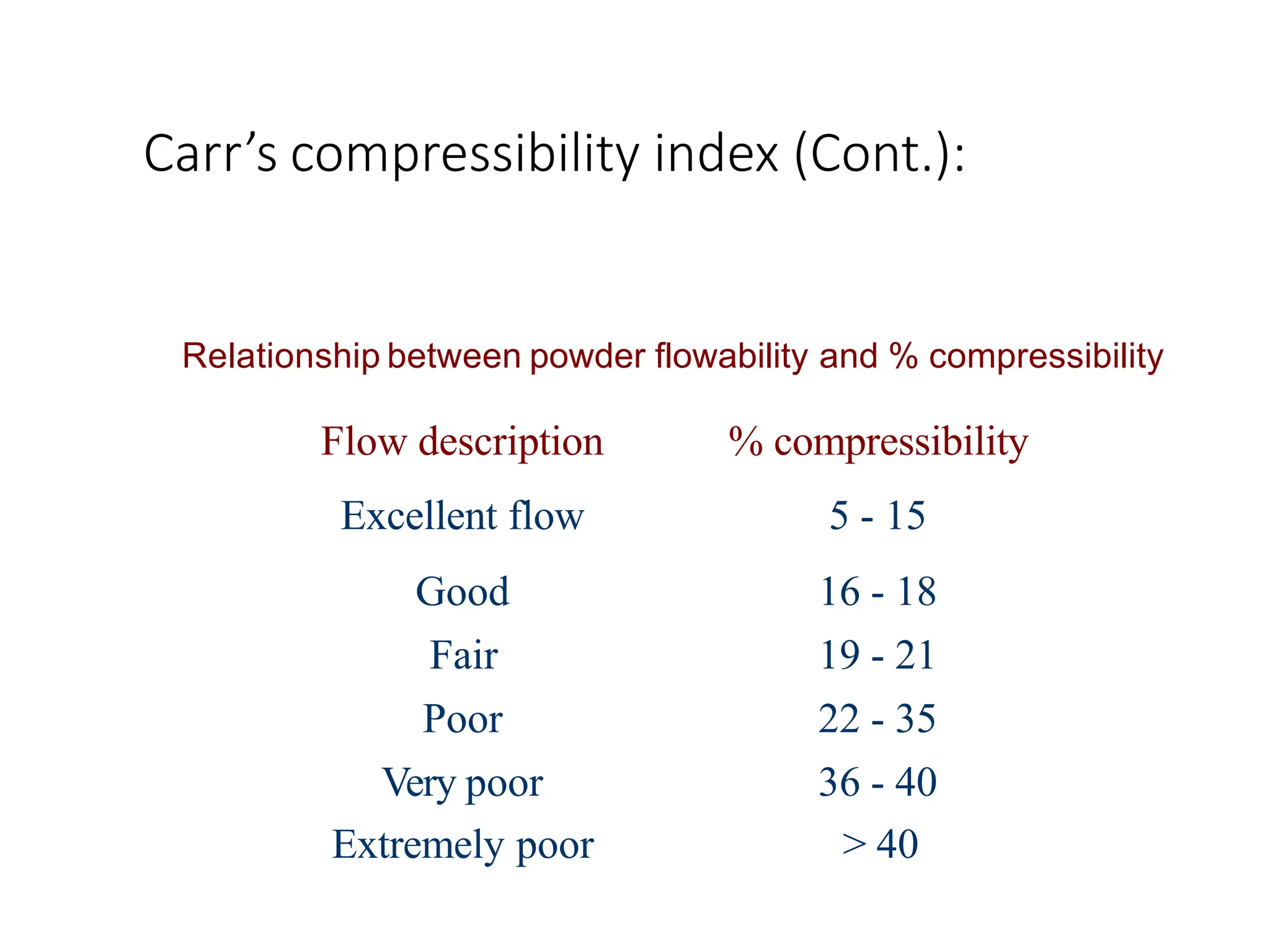
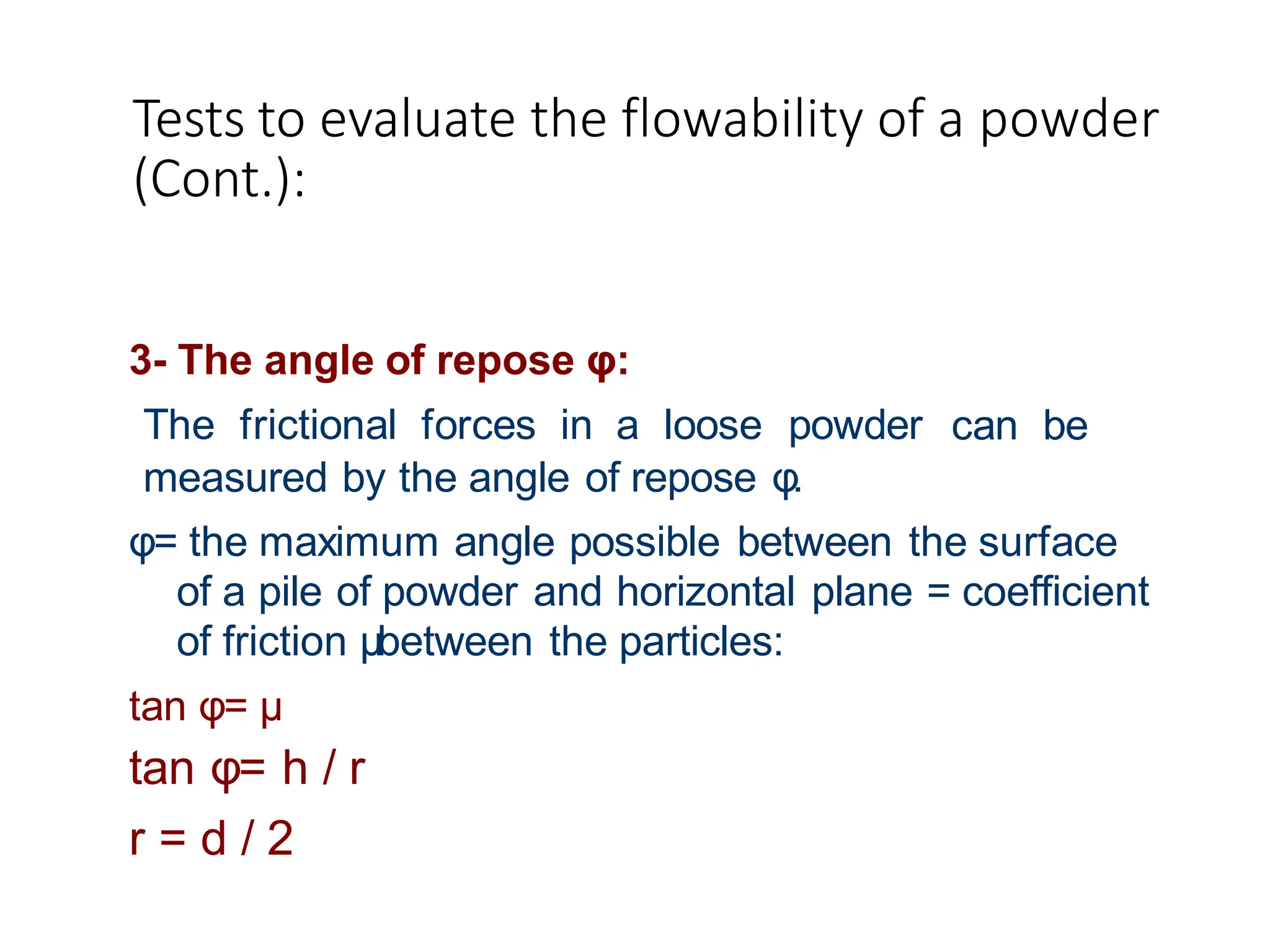
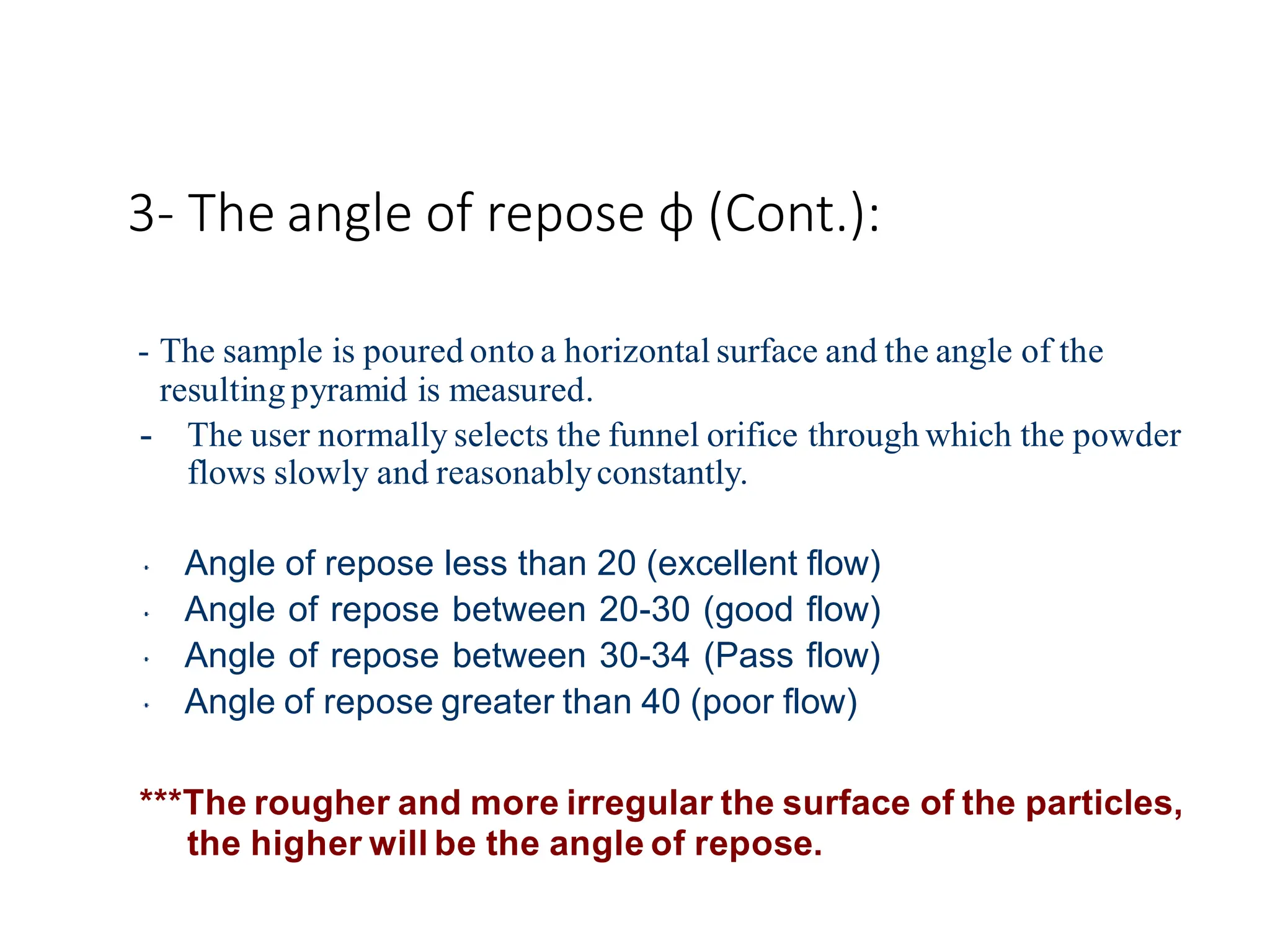
The document provides a comprehensive overview of micromeritics, focusing on the science and technology of small particles, particularly related to pharmacy and material science. It details the measurement and significance of particle size, characteristics, methods for size determination, as well as the influence of size on drug release, absorption, stability, and manufacturing. The document covers various techniques including optical microscopy, sieving, sedimentation, and particle volume measurement to assess particle size and distribution.