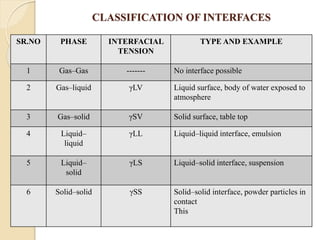
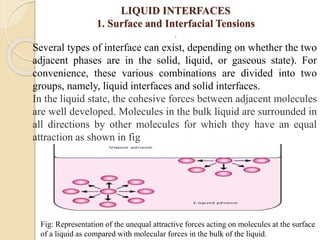
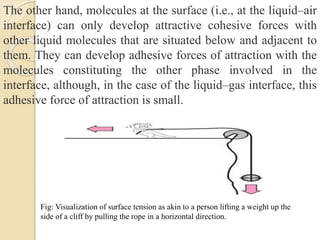
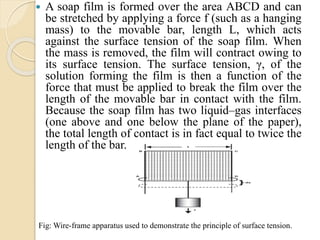
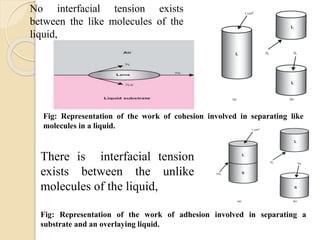
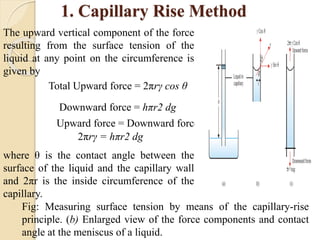
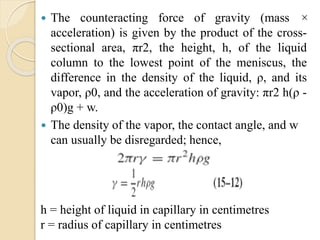
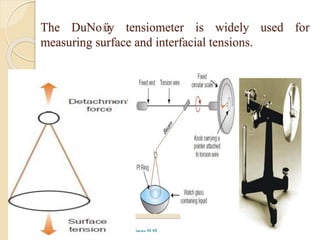
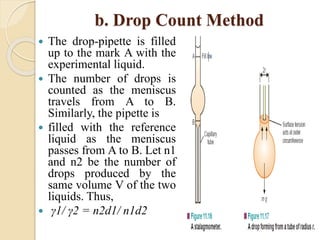
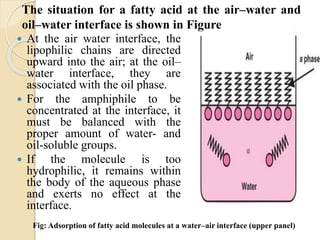
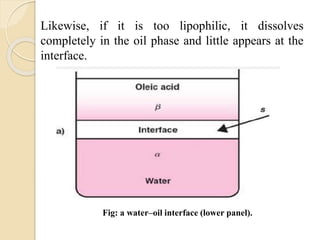
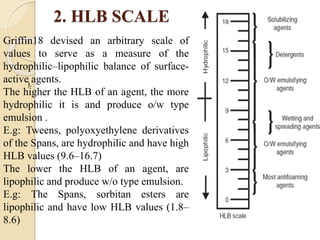
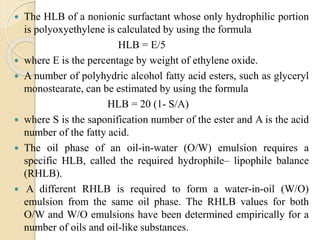
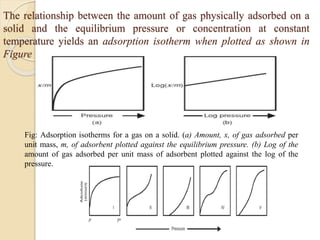
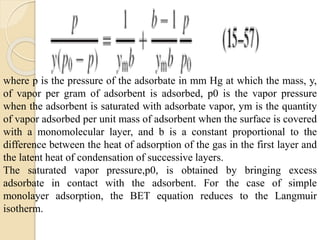
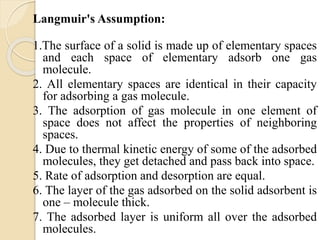
The document provides an in-depth discussion on surface tension, defining it as the force per unit length that counterbalances the inward pull on the surface of a liquid, with various types of interfacial tensions also addressed. It explains how surface tension is affected by adhesion and cohesion between different phases, and details various methods for measuring these tensions, including capillary rise and the Du Noüy ring method. Additionally, it covers concepts of adsorption at interfaces, including the impact of surface-active agents and the hydrophilic-lipophilic balance.