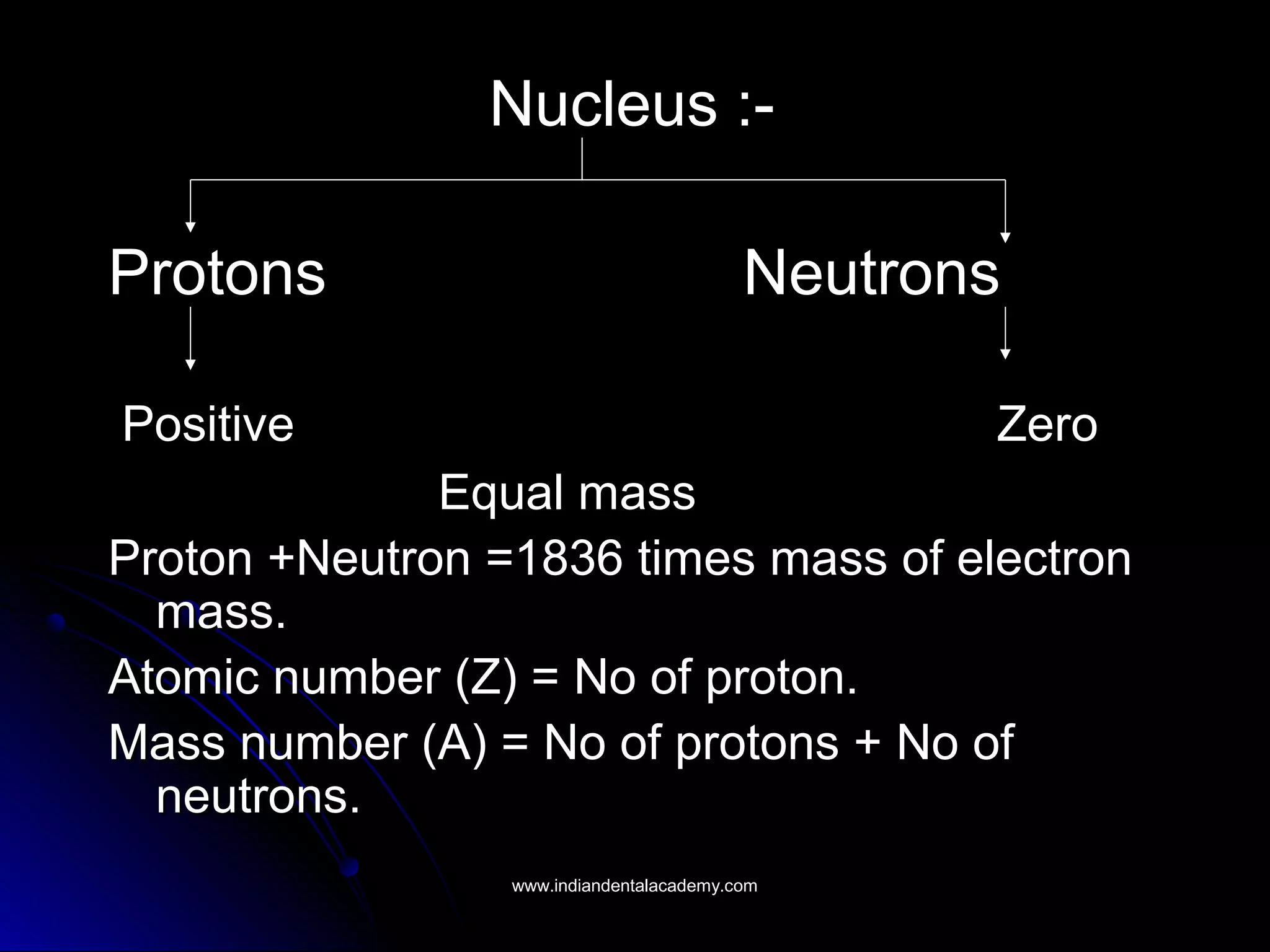
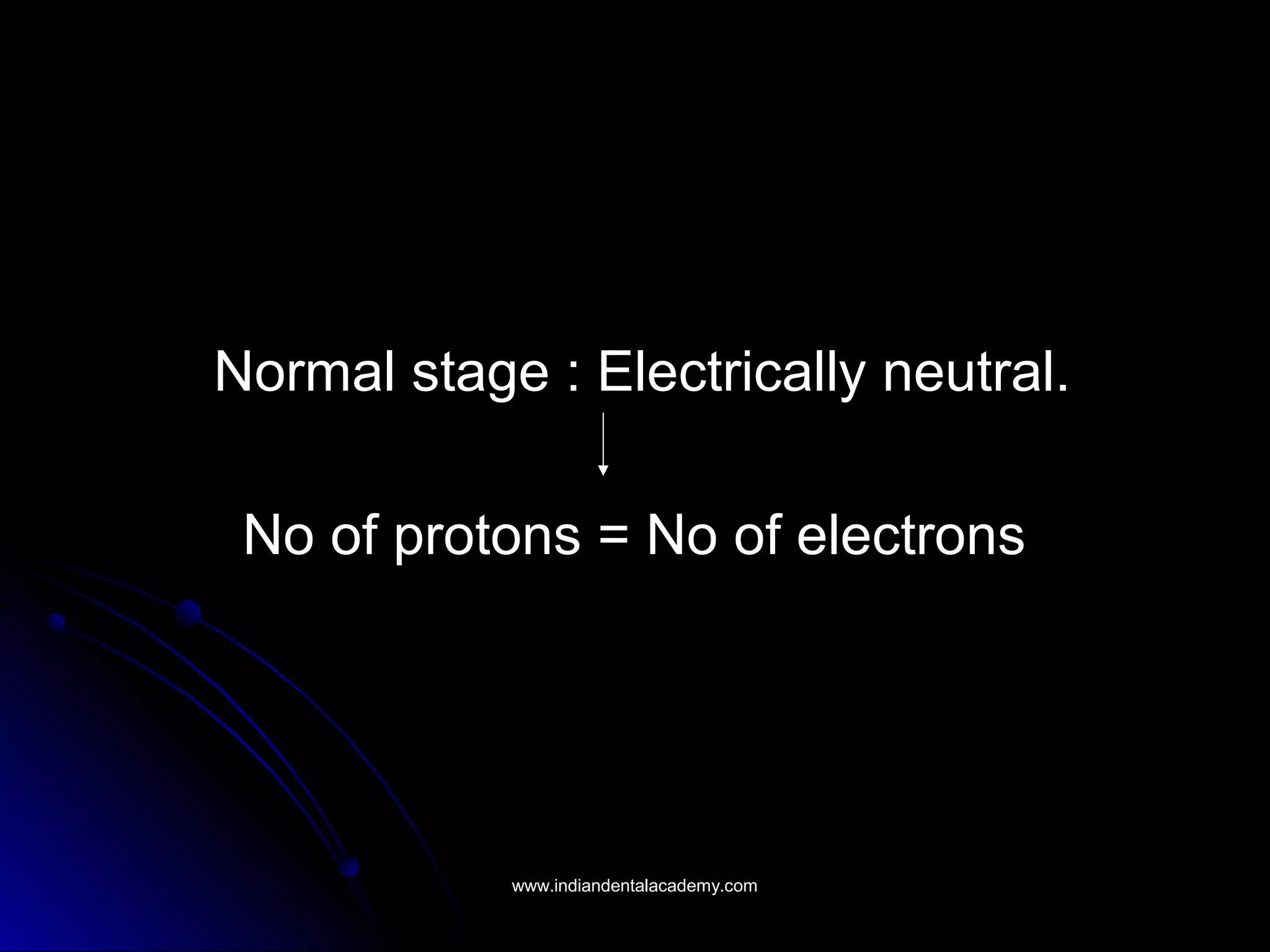
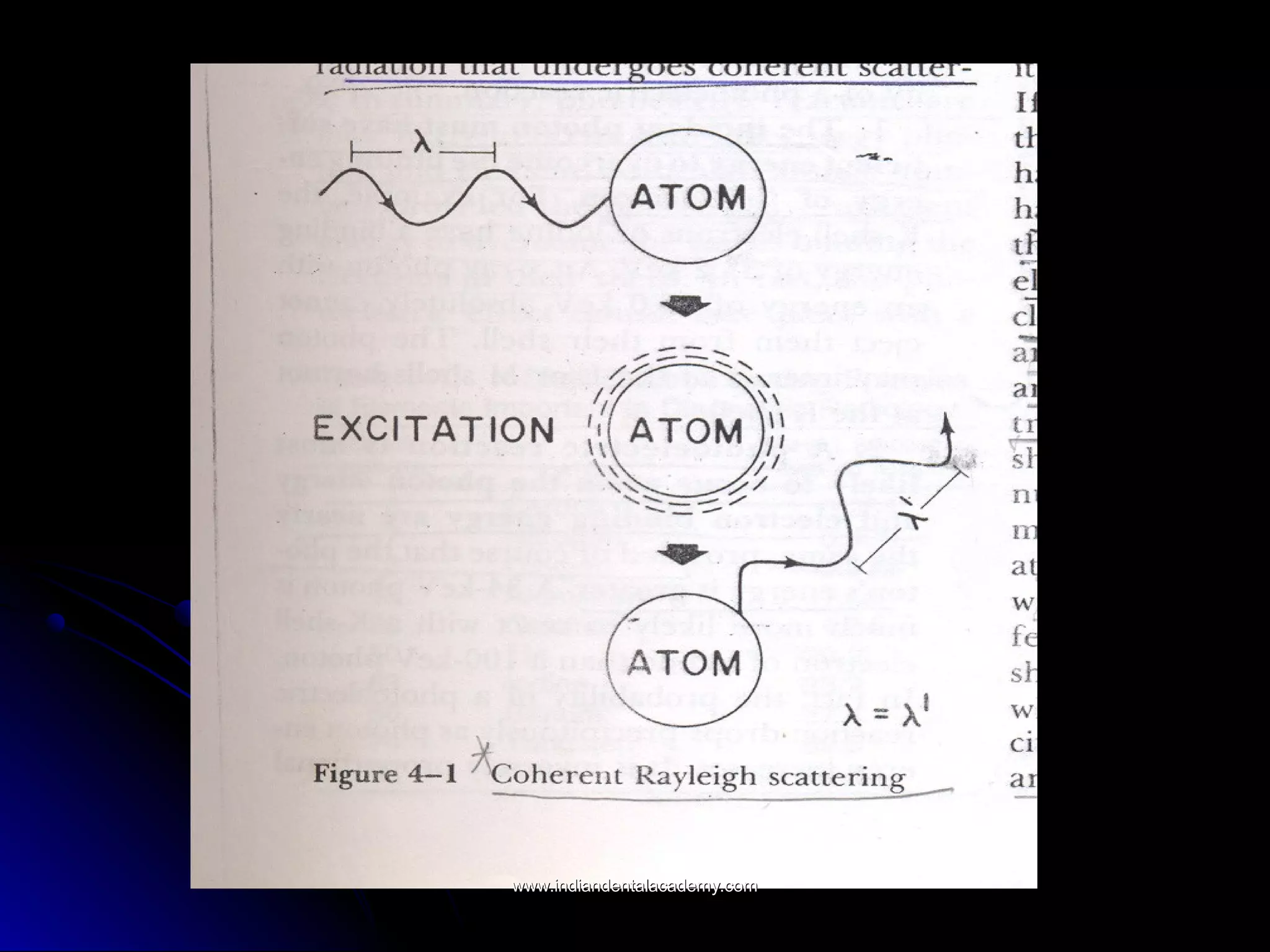
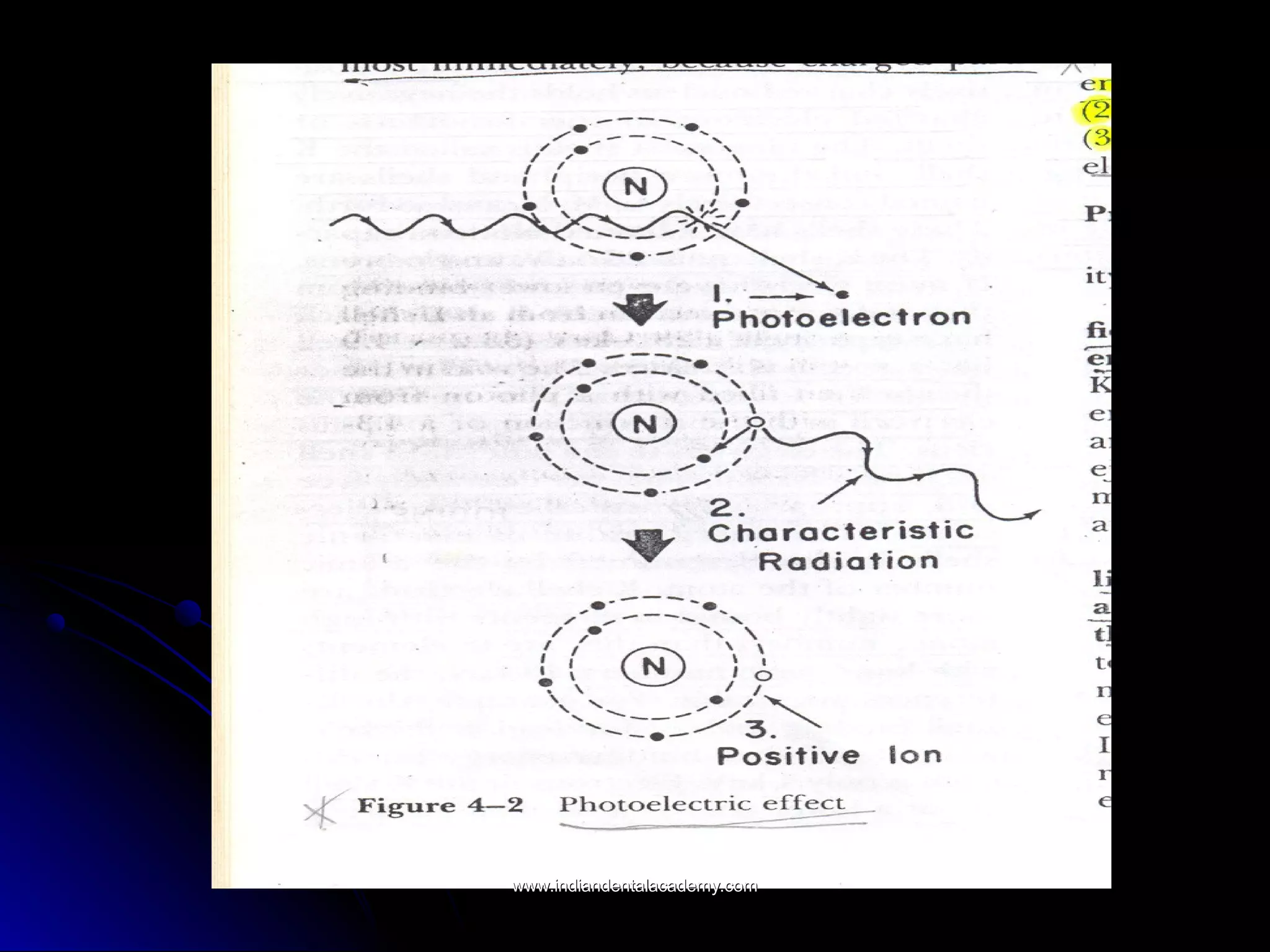
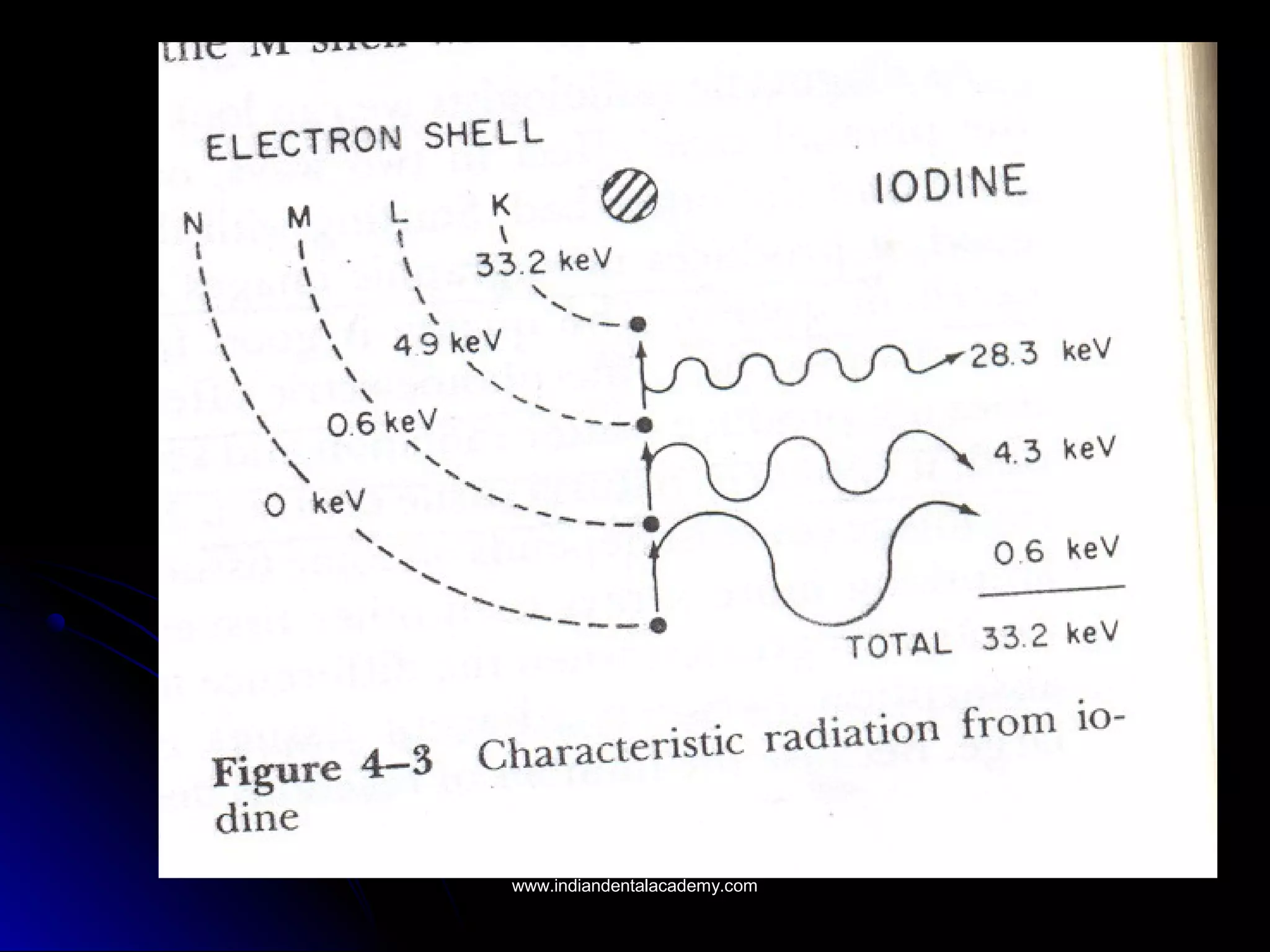
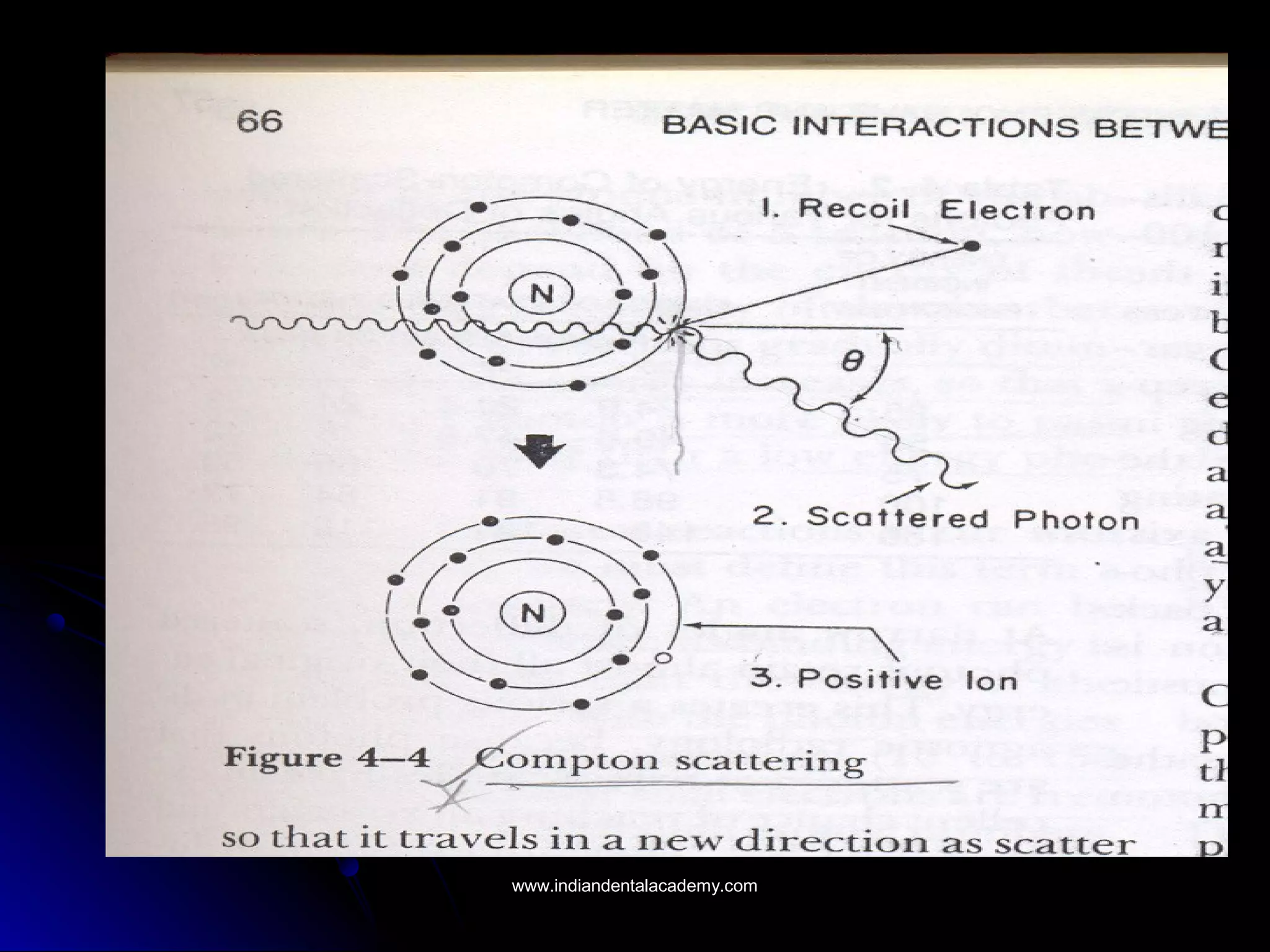
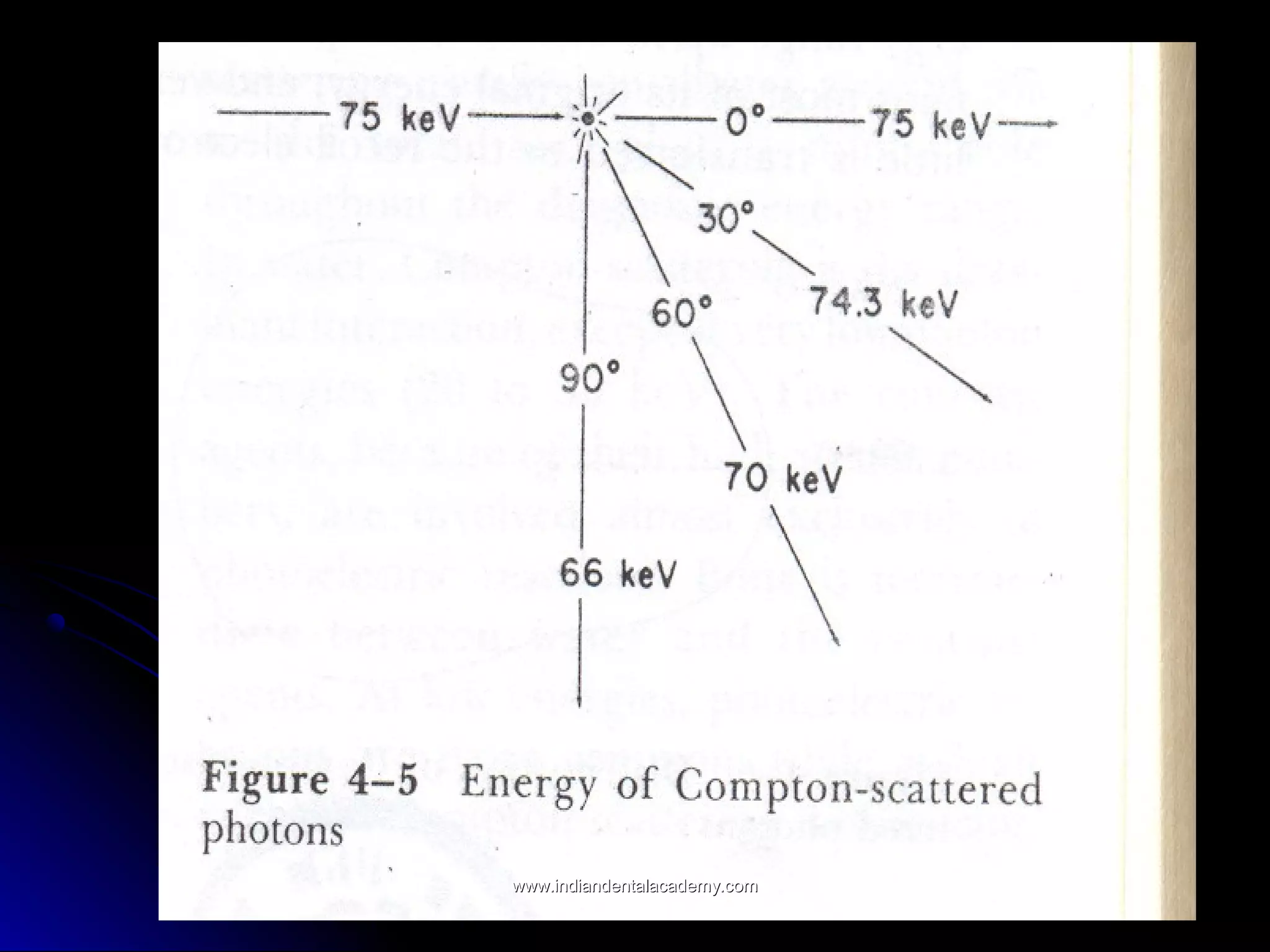
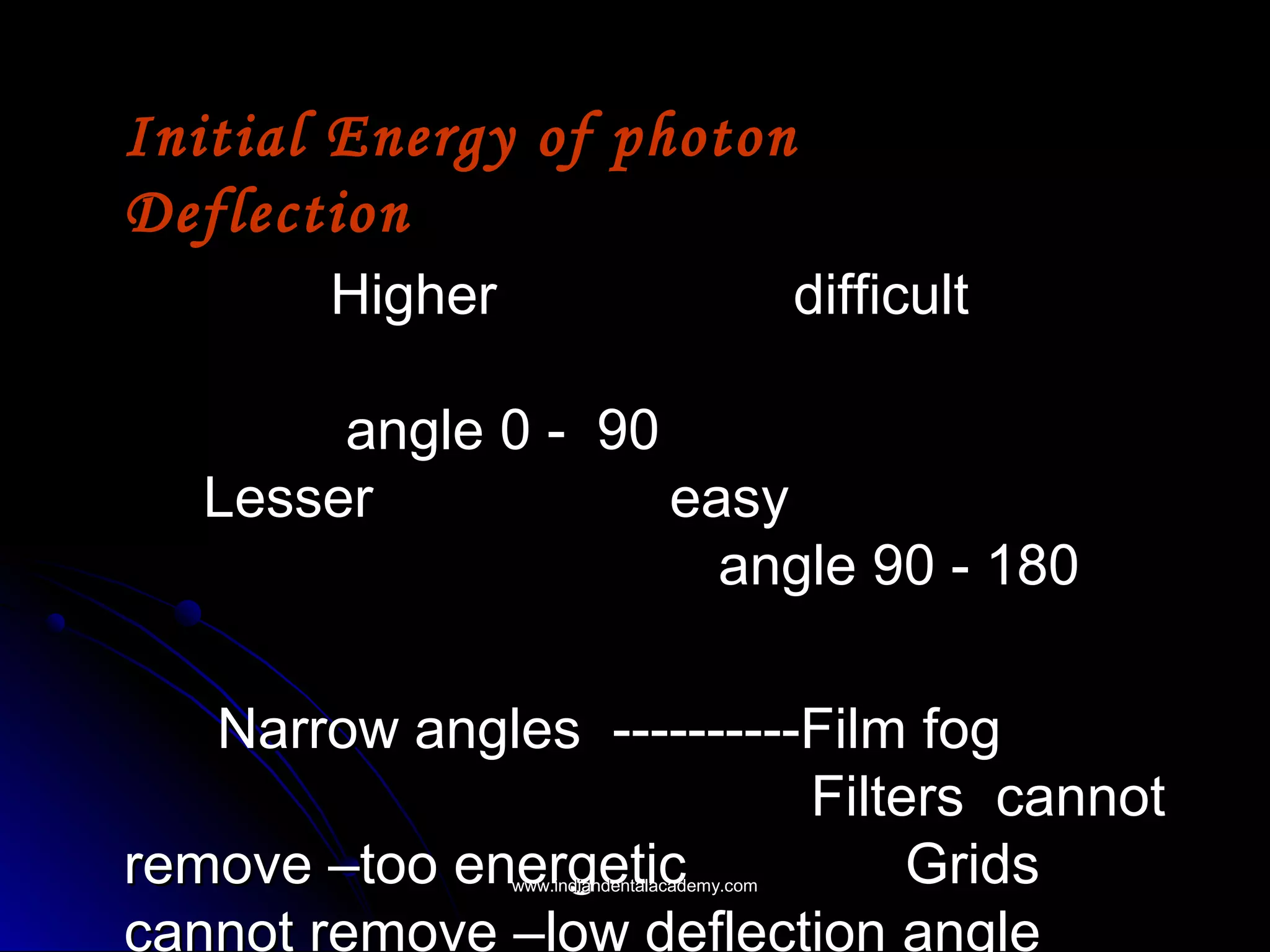
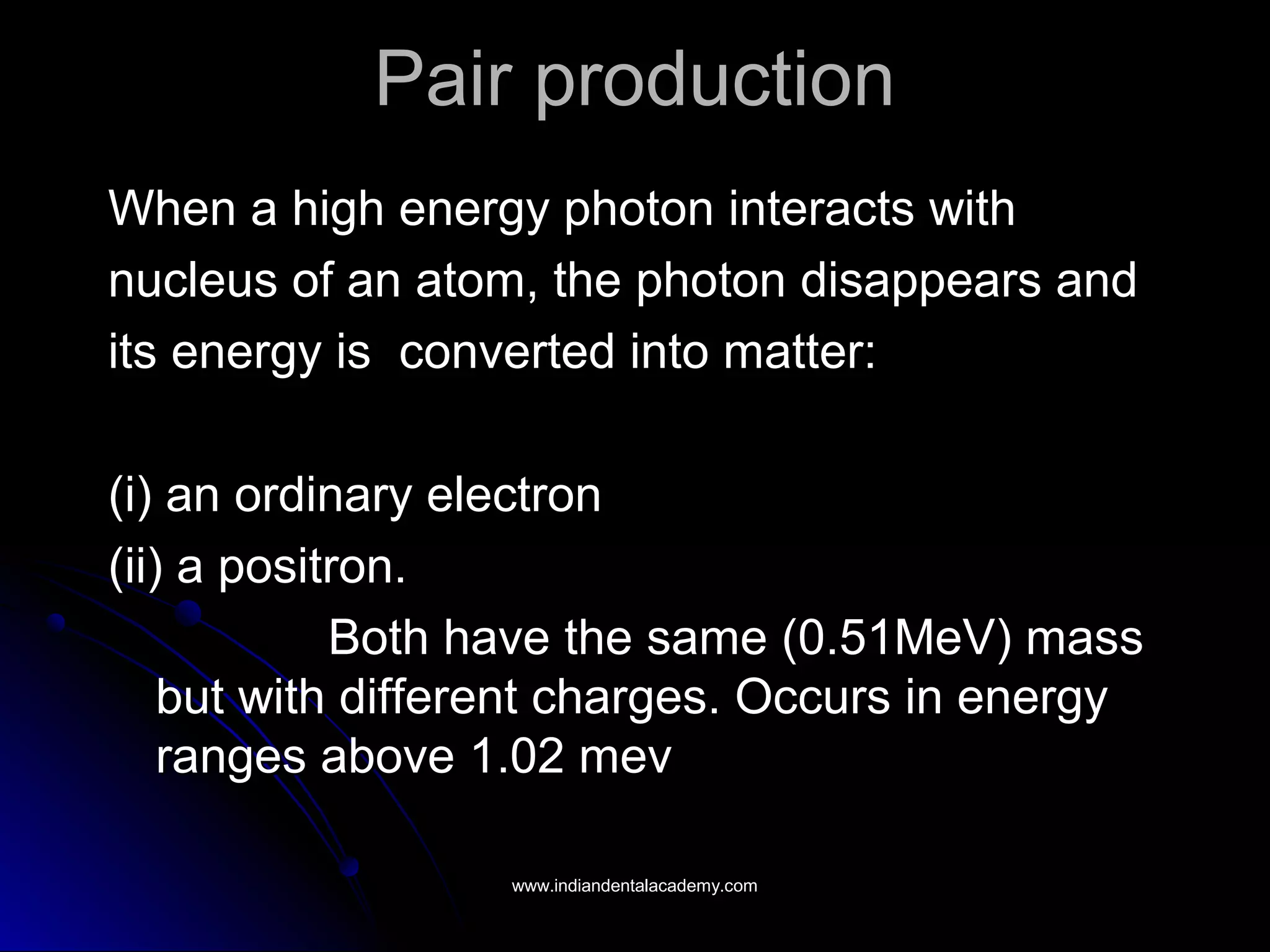
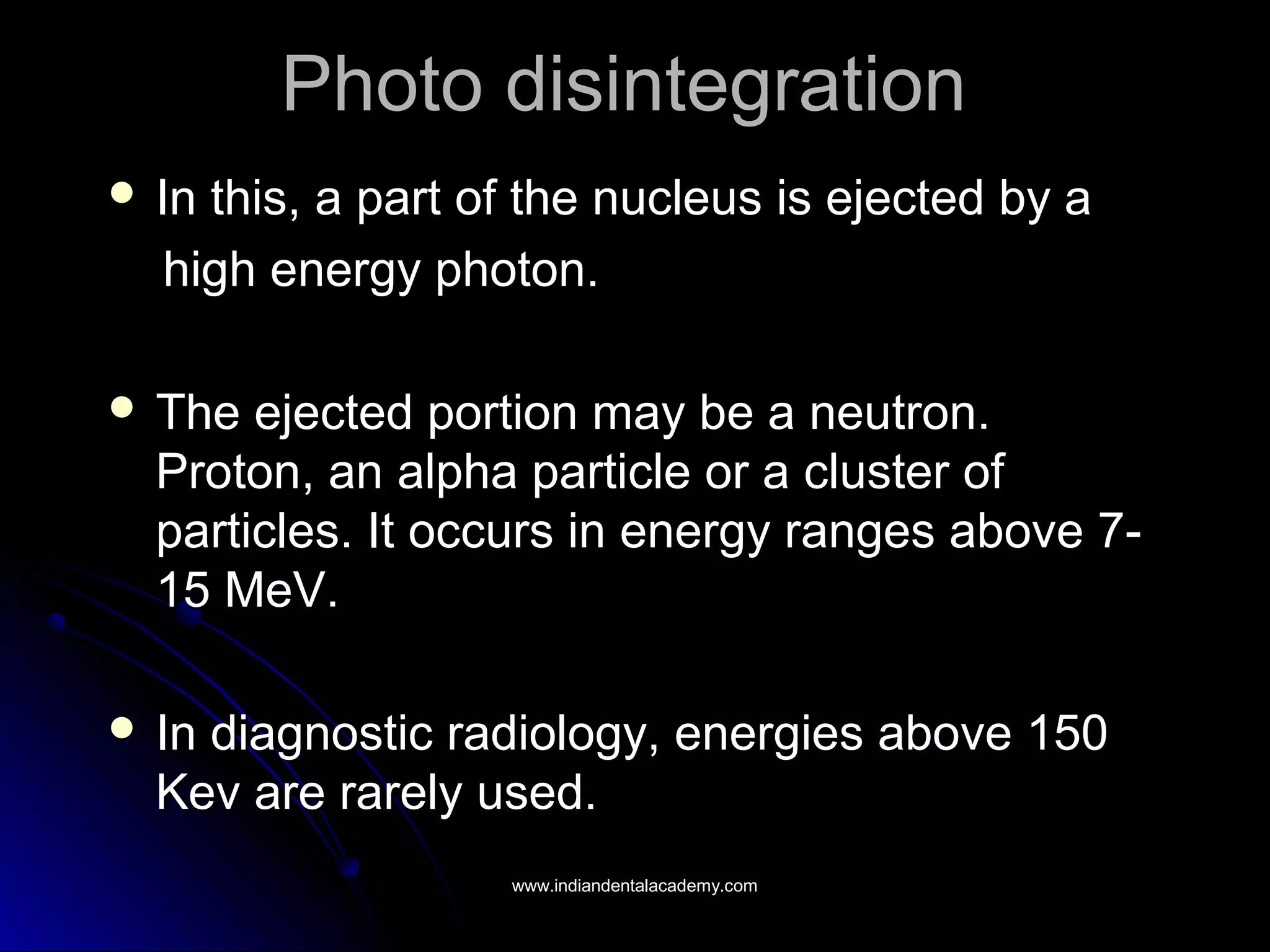
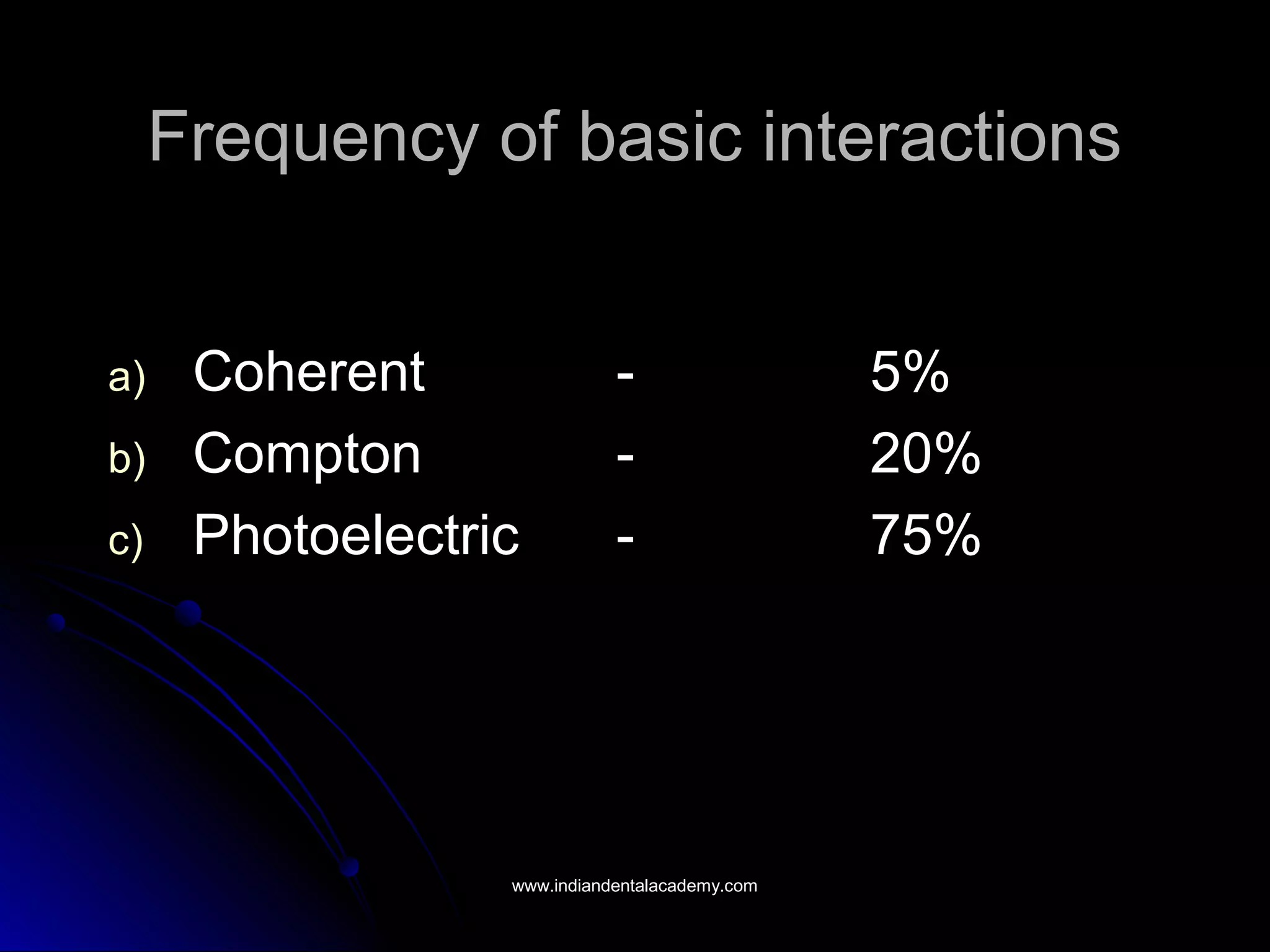
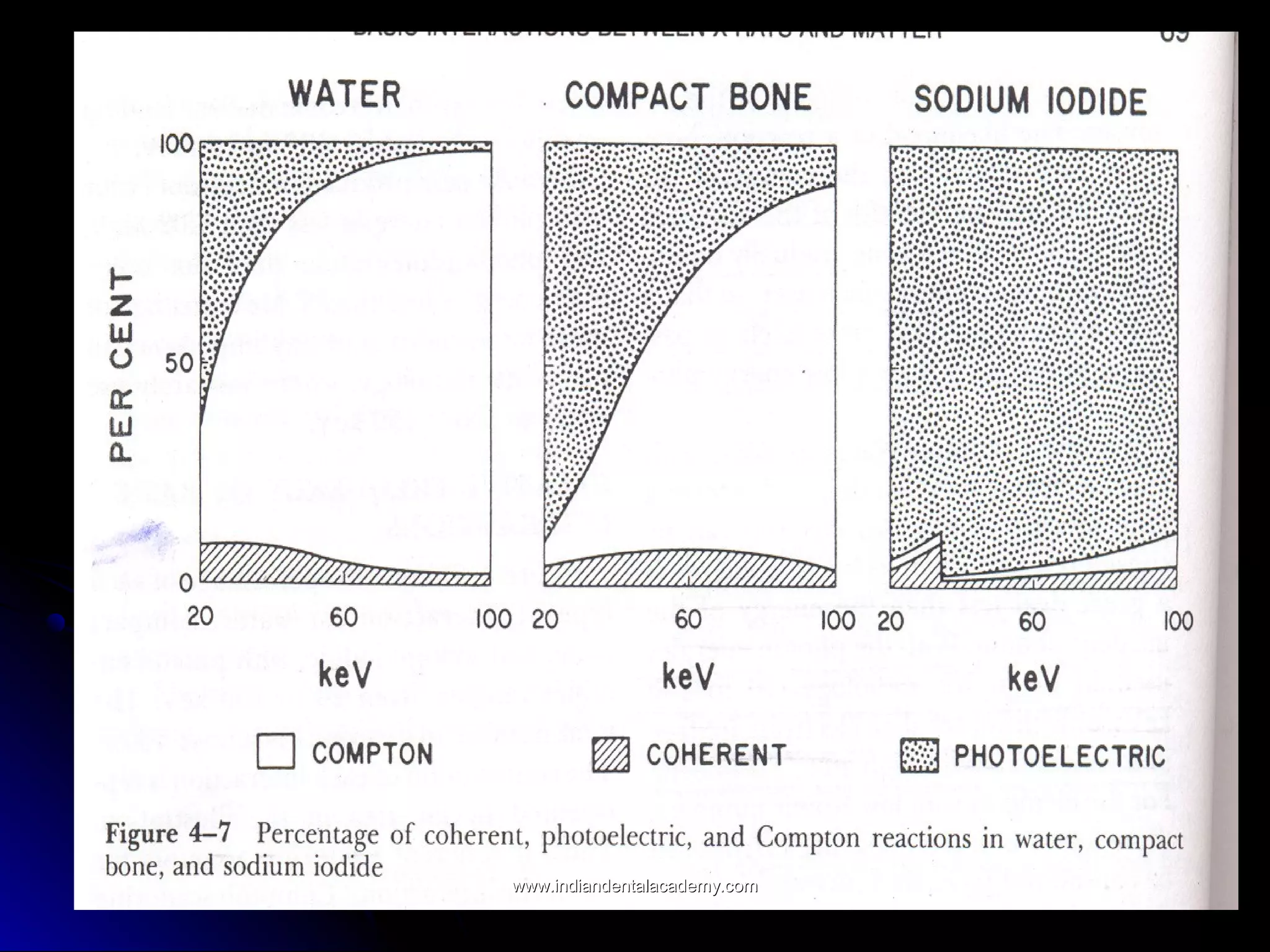
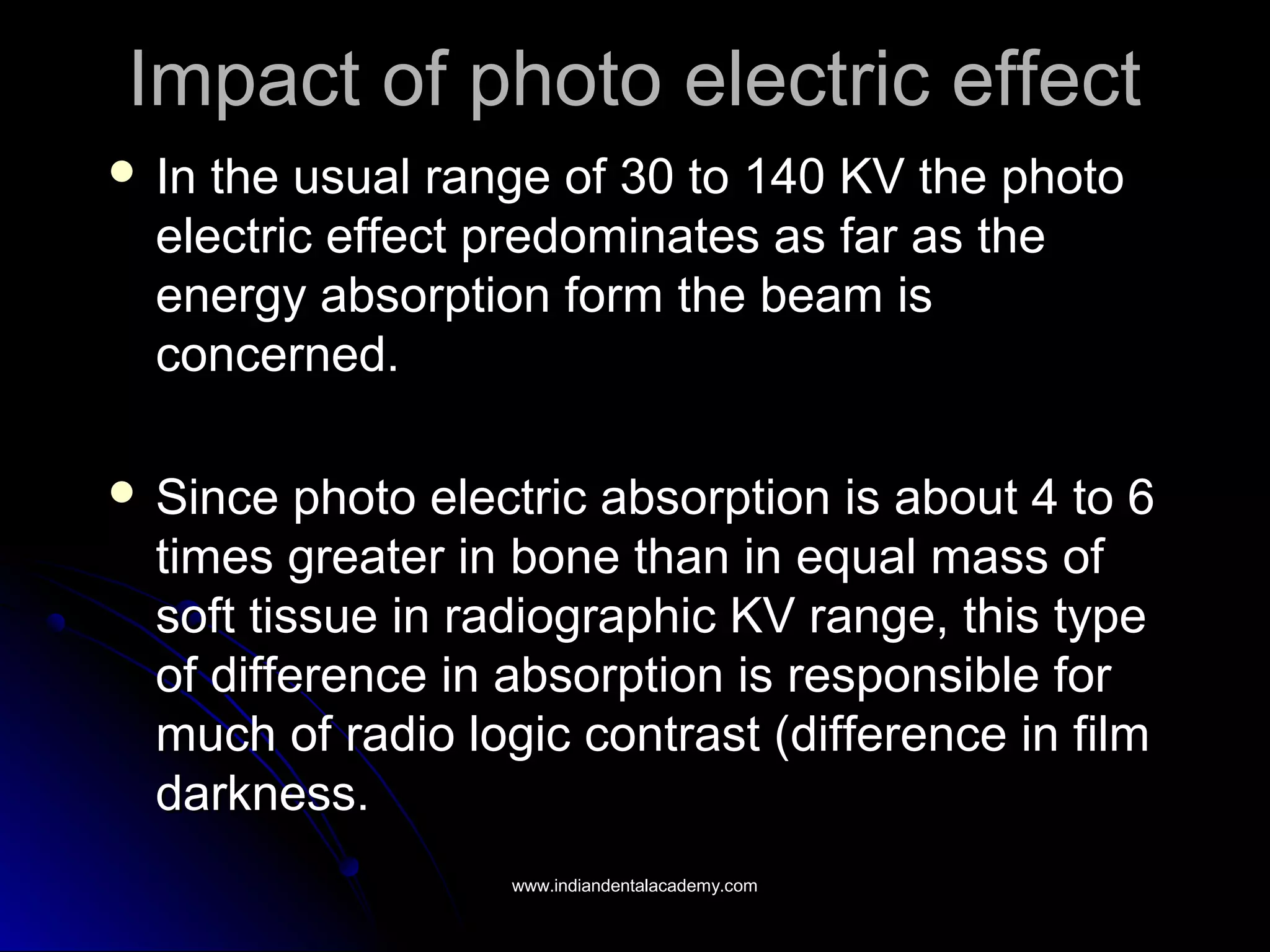
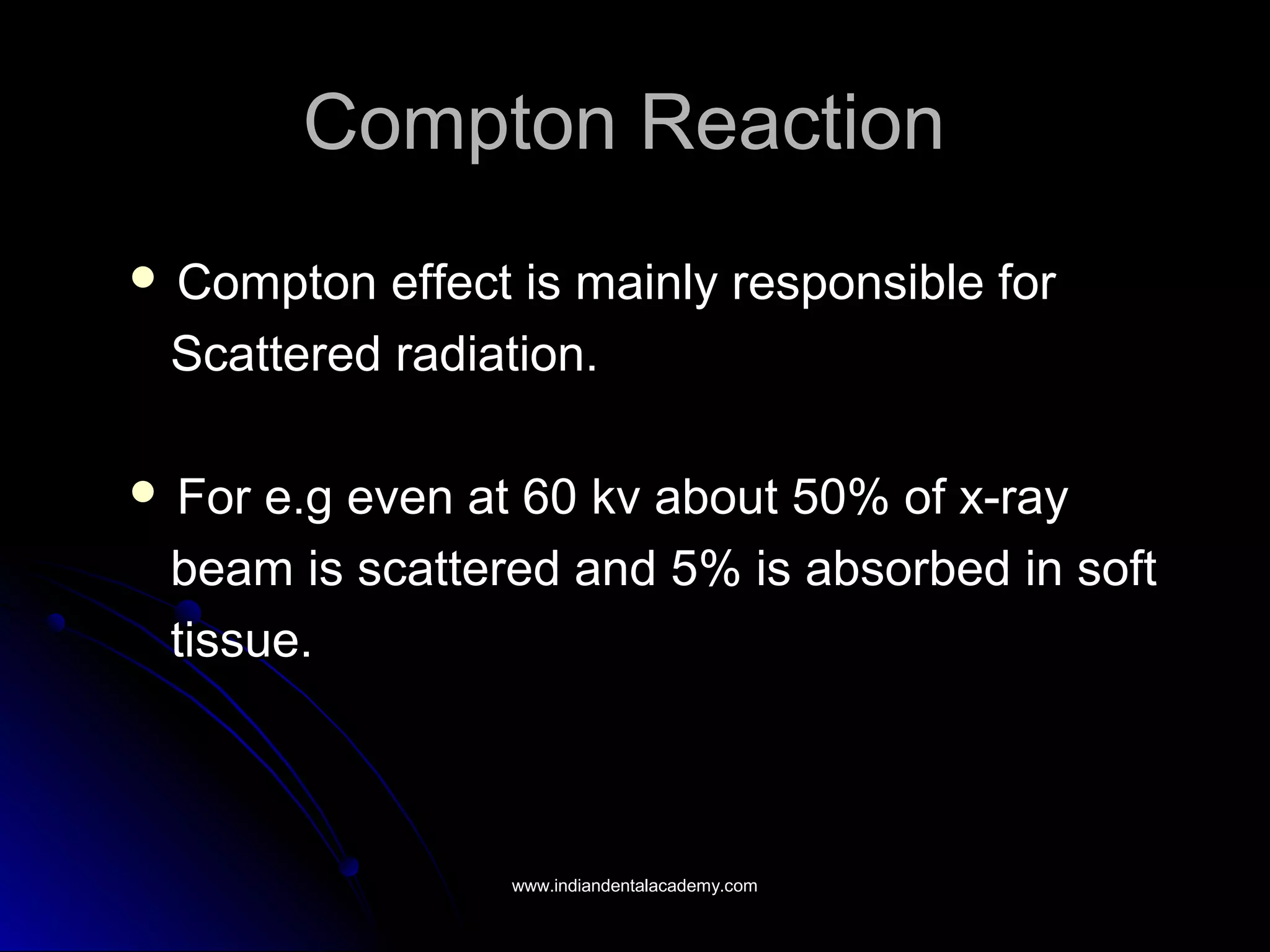
The document explains the fundamental interactions between x-rays and matter, detailing concepts like binding energy, photoelectric effect, and Compton reaction. It describes how x-rays can be absorbed or scattered, affecting image quality in radiography. Additionally, it outlines types of radiation, their production processes, and the advantages and disadvantages of various x-ray interactions.