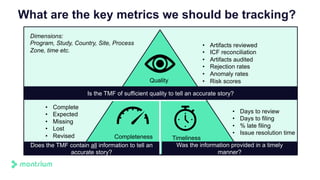
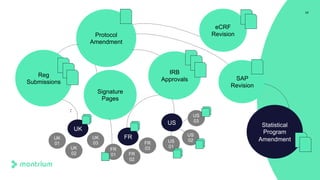
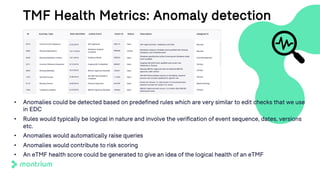
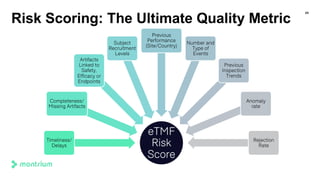
The document is a webinar agenda and content overview focused on improving metrics and completeness reporting in TMF (Trial Master File) management. Key topics include current flaws in completeness and timeliness measurements, the importance of integrating process modeling and data management techniques to enhance quality metrics, and the potential for risk scoring to improve inspection readiness. The speaker, Paul Fenton from Montrium, emphasizes the need for a more accurate and data-driven approach to TMF management to ensure compliance and effective clinical trial operation.