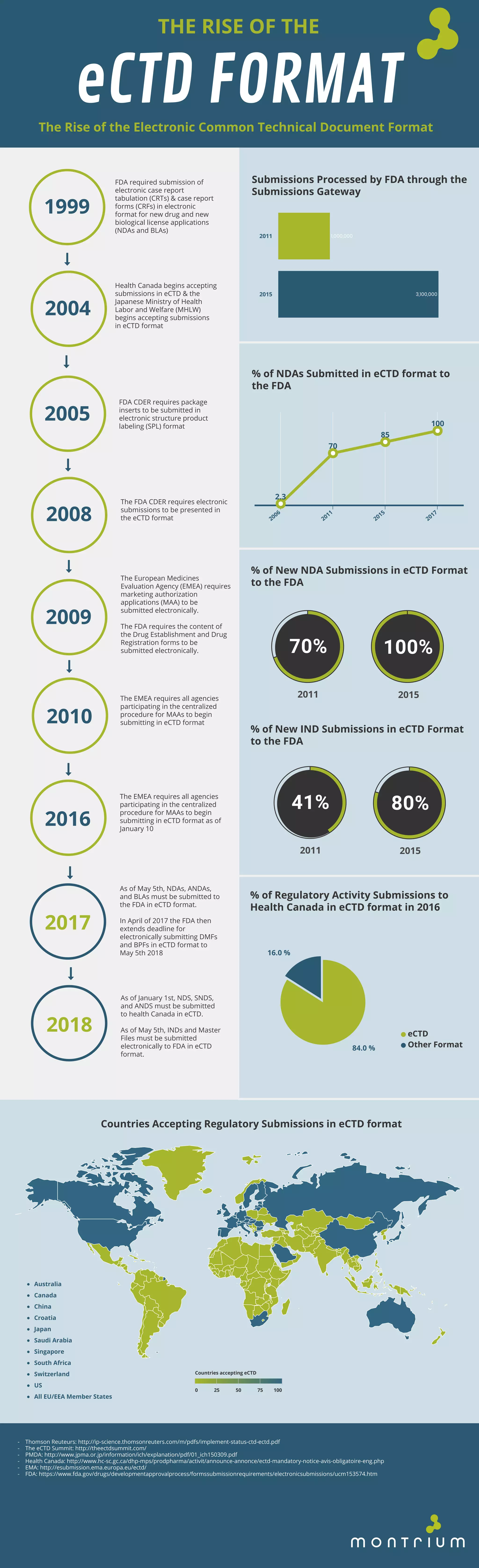
The document outlines the development and adoption of the electronic common technical document (eCTD) format from 1999 to 2018, detailing its mandatory use by various health authorities like the FDA, Health Canada, and the EMEA for drug submissions. It highlights the increasing percentage of submissions in eCTD format and the deadlines set for various types of applications, including NDAs and BLAs. By 2018, eCTD format became the standard for regulatory submissions across multiple countries.