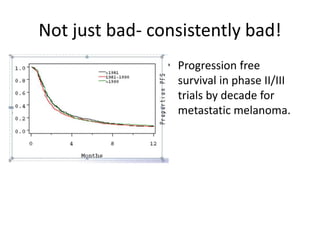
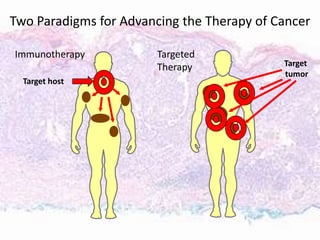
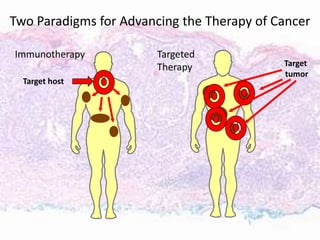
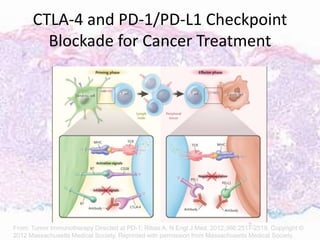
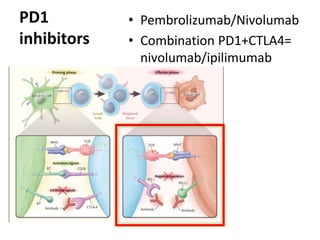
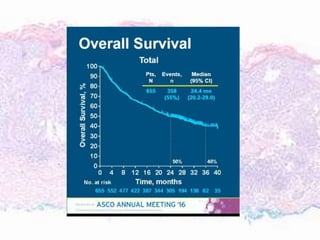
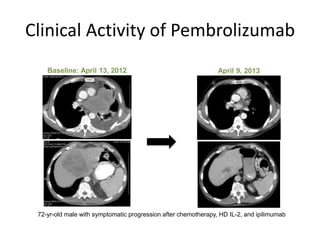
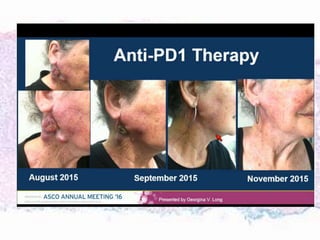
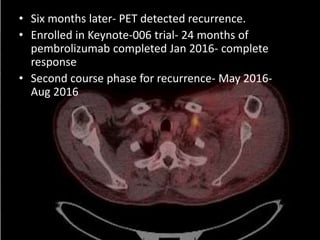
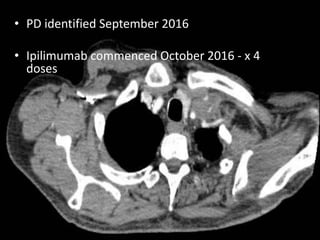
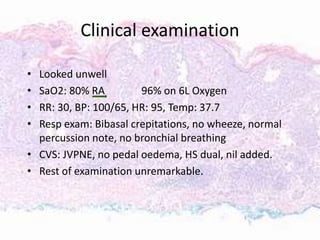
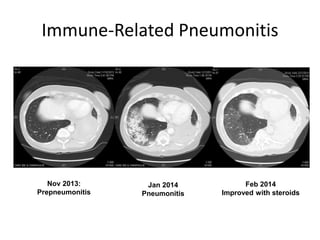
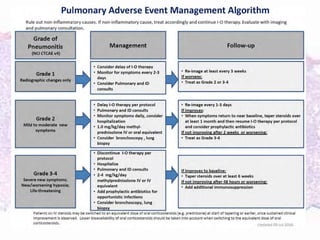
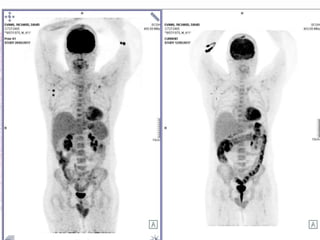
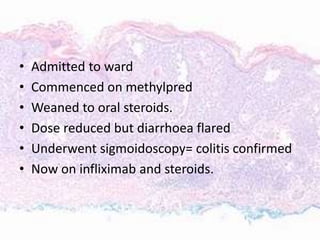
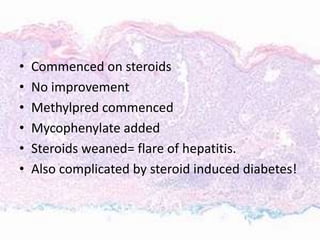
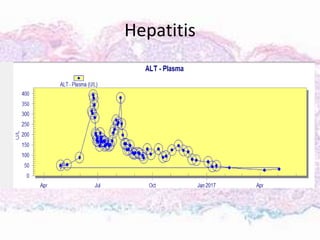
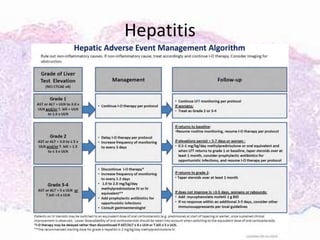
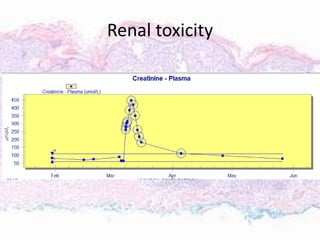
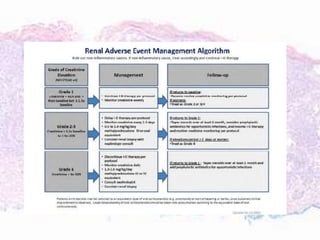
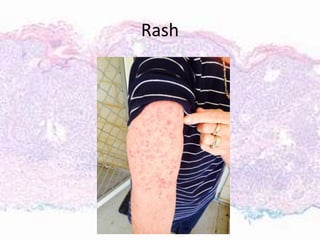
This document discusses immunotherapy for cancer treatment and its promises as well as pitfalls. It provides examples of how immunotherapy has been successfully used to treat metastatic melanoma but can also cause immune-related side effects involving the thyroid, endocrine system, lungs, gut, liver, kidneys and skin. The document presents three case studies of patients who developed pneumonitis, colitis, and hepatitis, respectively, while undergoing immunotherapy and required treatment with steroids and other immunosuppressants. Management of immune-related toxicity from cancer immunotherapy requires specialist input.