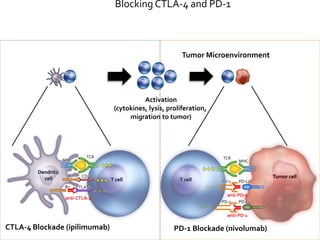
This document provides an overview of immunotherapy and how it works to treat cancer. It discusses the different types of T cells like CD4+ helper T cells and CD8+ cytotoxic T cells. It describes antigen presenting cells and their role in activating T cells. It explains how tumors evade immune surveillance and the factors that allow this. It discusses different immunotherapy approaches like blocking the CTLA-4 and PD-1 pathways with drugs like ipilimumab and nivolumab. Clinical trial results are summarized that show improved survival with these immunotherapies compared to chemotherapy in cancers like melanoma. Combination approaches are also discussed.