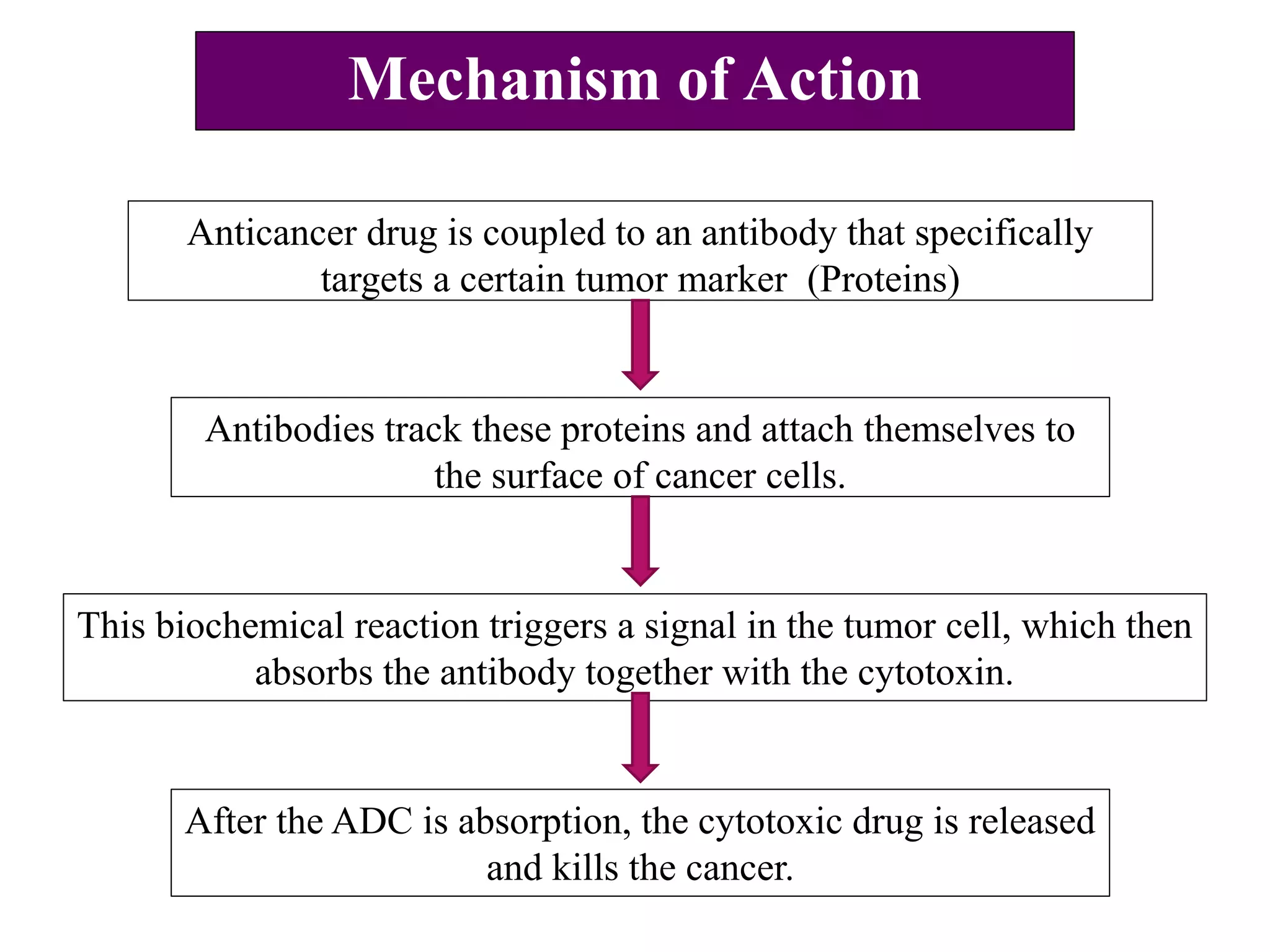
The document presents an overview of targeted anti-cancer therapies, detailing types of cancer, biomarkers, and specific therapeutic approaches like antibody drug conjugates (ADCs), bispecific monoclonal antibodies, and immuno-checkpoint inhibitors. It explains mechanisms of action, advantages, and provides examples of approved therapies, emphasizing the ability of these treatments to selectively target cancer cells while reducing side effects. Various references are cited to support the information provided.