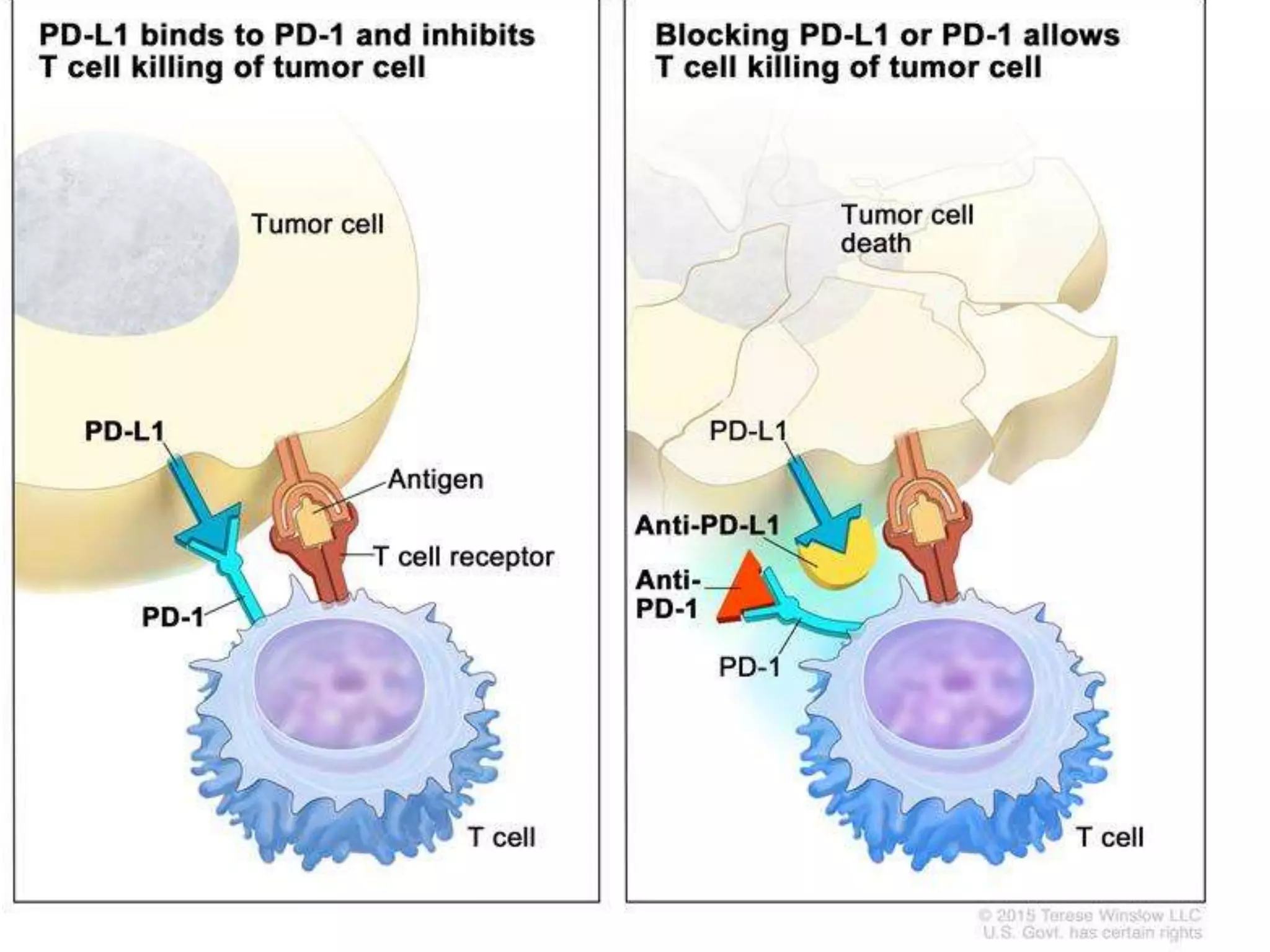
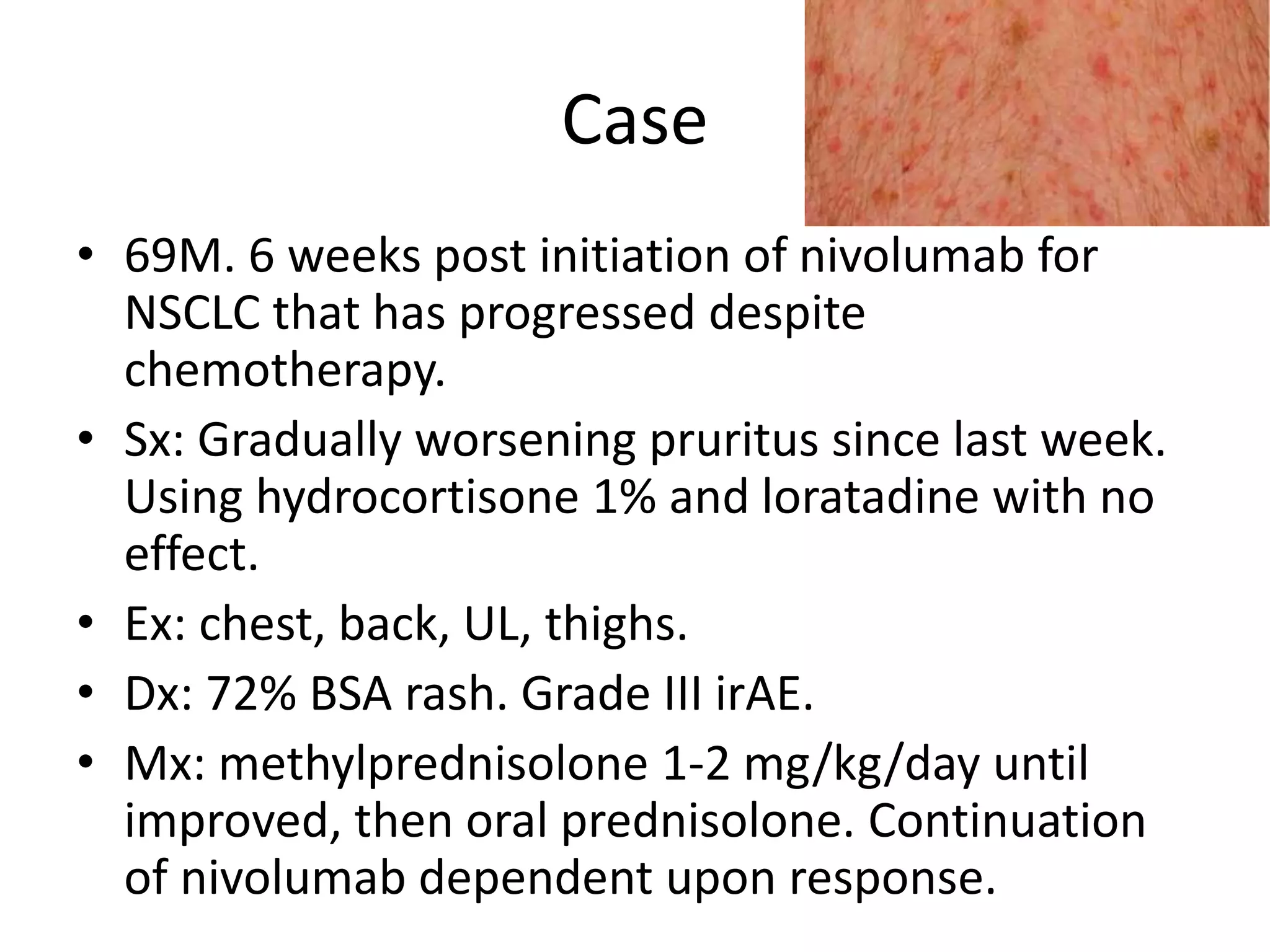
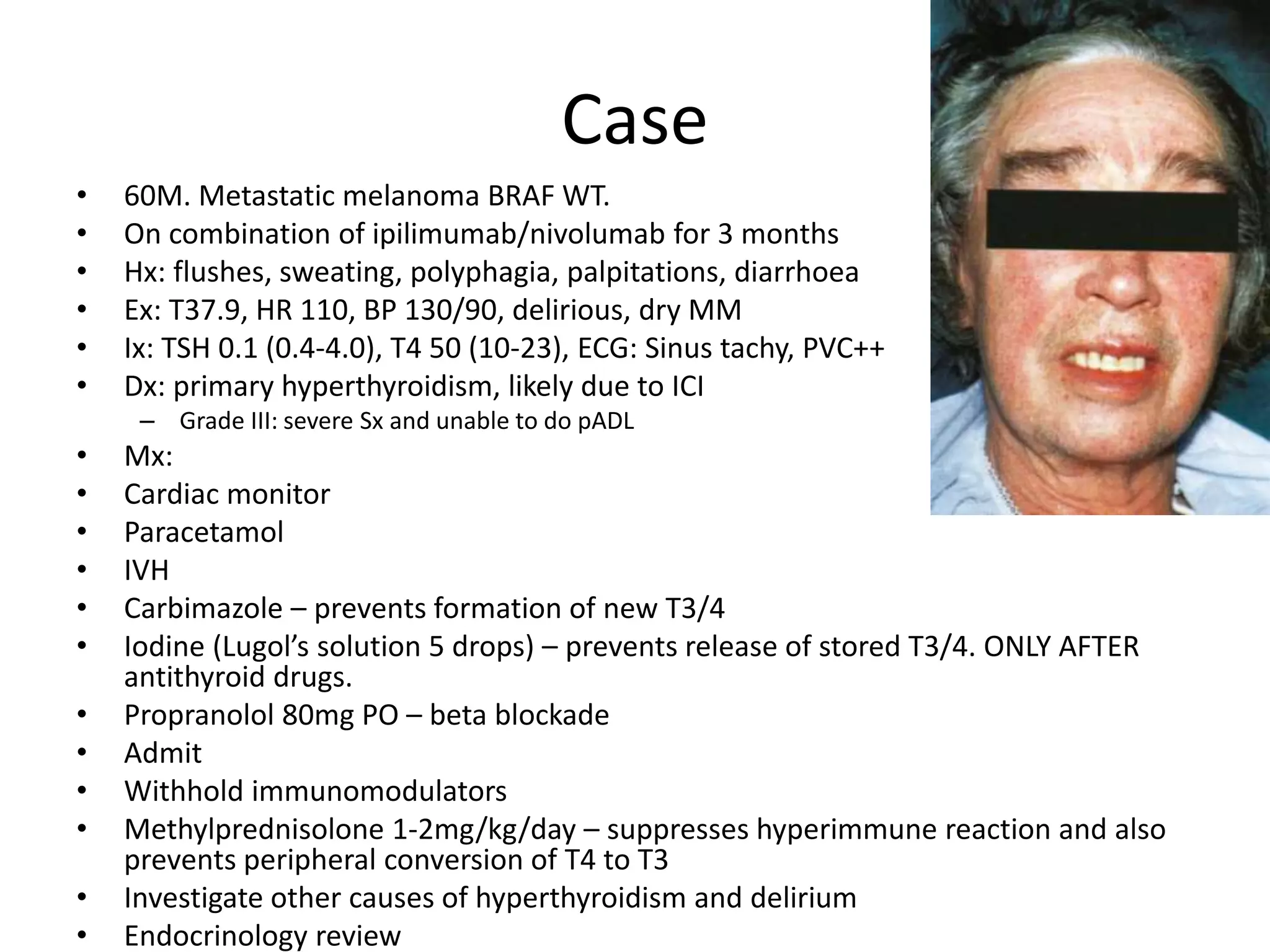
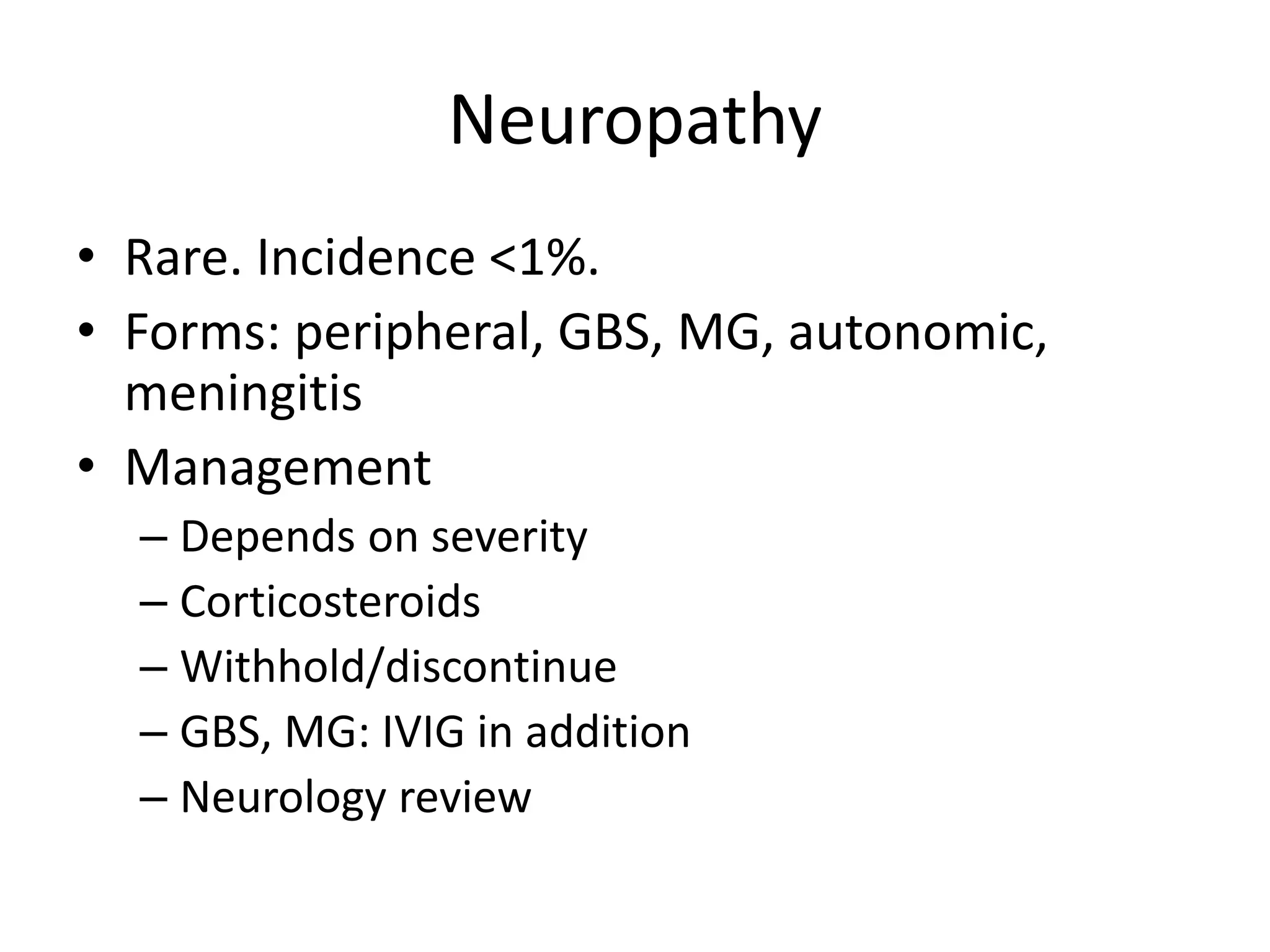
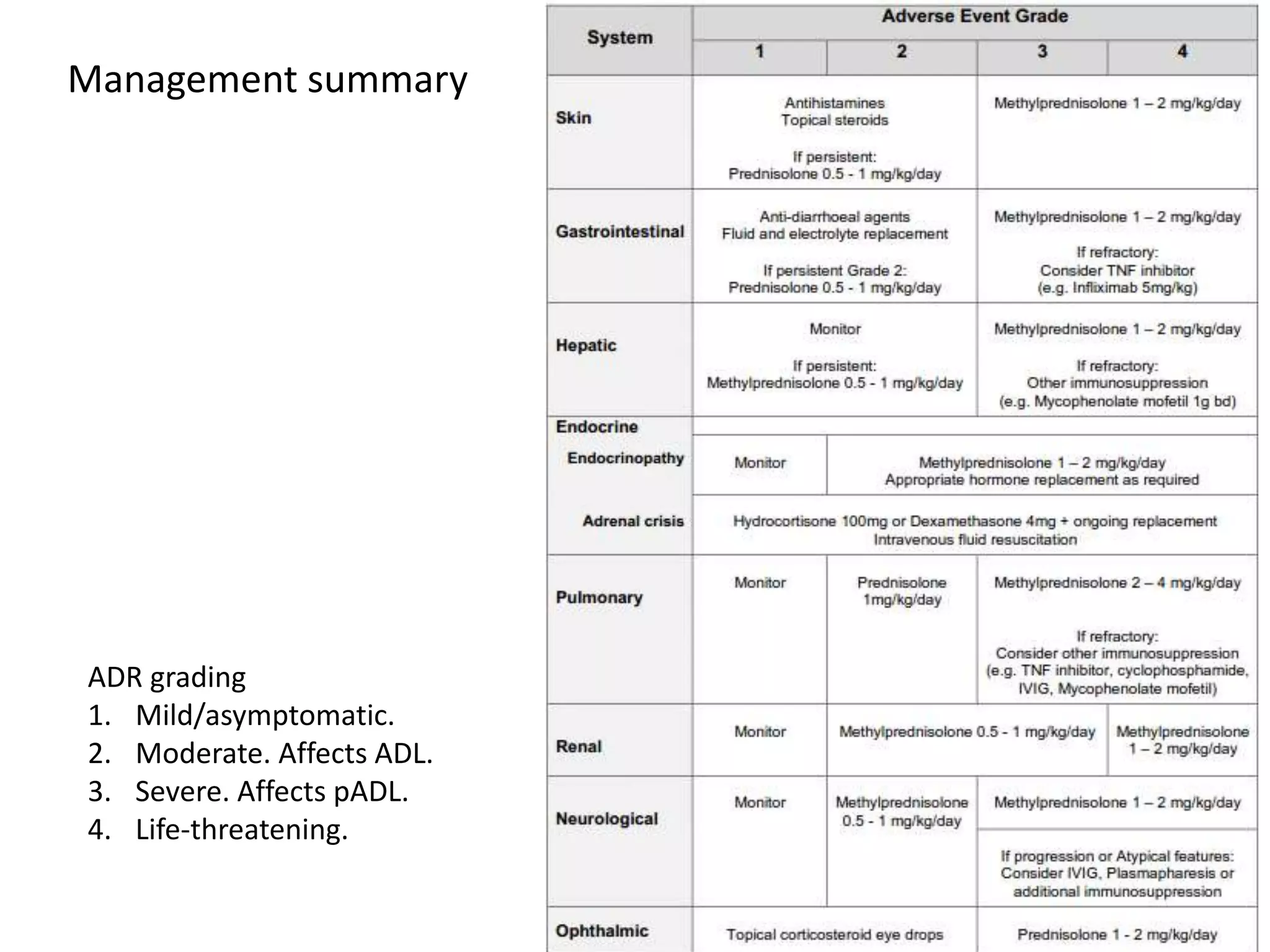
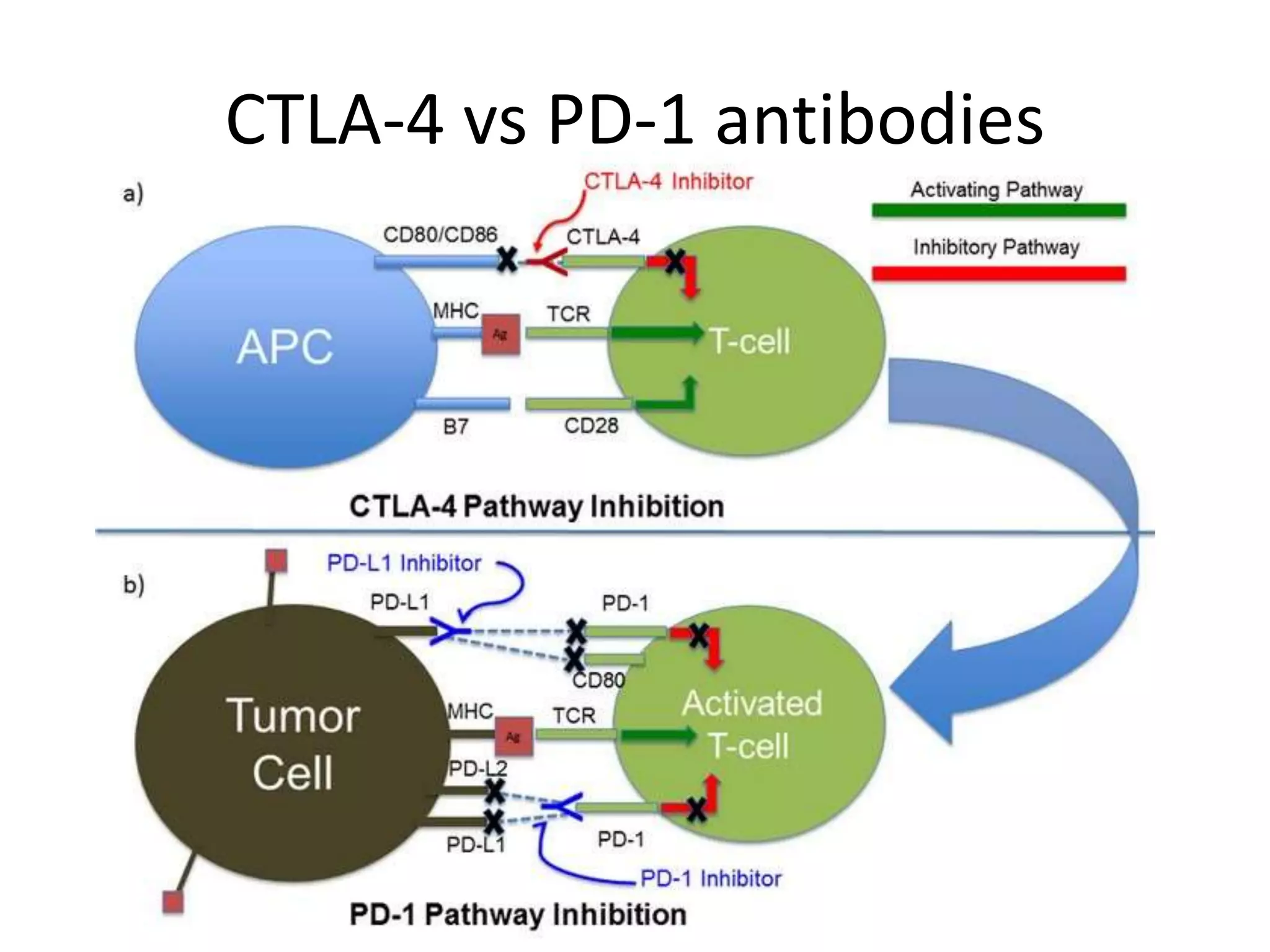
Immune checkpoint inhibitors can cause immune-related adverse events by removing inhibitory checkpoints and excessively activating the immune system. Common adverse effects include dermatitis in over 50% of patients, enterocolitis in under 20%, hepatitis in under 10%, and endocrinopathies. Symptoms usually onset several months after treatment. Management involves stopping the immune checkpoint inhibitor and administering corticosteroids in most cases. Life-threatening hyperimmunity is possible so consulting the oncologist and other specialists is important.