IB Chemistry on Redox, Oxidation states and Oxidation number
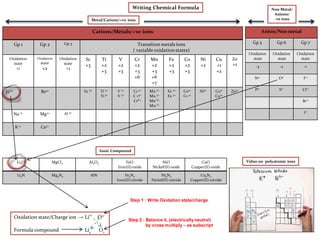
- 1. Cations/Metals/+ve ions Gp 1 Gp 2 Gp 3 Transition metals ions ( variable oxidation states) Oxidation state +1 Oxidation state +2 Oxidation state +3 Sc +3 Ti +2 +3 V +2 +3 Cr +2 +3 +6 Mn +2 +3 +6 +7 Fe +2 +3 Co +2 +3 Ni +2 Cu +1 +2 Zn +2 Li 1+ Be2+ Sc 3+ Ti 2+ Ti 3+ V 2+ V 3+ Cr 2+ C r3+ Cr6+ Mn 2+ Mn 3+ Mn 6+ Mn 7+ Fe 2+ Fe 3+ Co2+ Co 3+ Ni2+ Cu1+ Cu2+ Zn2+ Na 1+ Mg2+ Al 3+ K 1+ Ca2+ Anion/Nonmetal Gp 5 Gp 6 Gp 7 Oxidation state Oxidation state Oxidation state -3 -2 -1 N3- O2- F-1 P3- S2- CI-1 Br-1 I-1 Metal/Cations/+ve ions Non Metal/ Anions/ -ve ions Ionic Compound Li2O MgCI2 Al2O3 FeO Iron(II) oxide NiO Nickel(II) oxide CuO Copper(II) oxide Li3N Mg3N2 AlN Fe3N2 Iron(II) nitride Ni3N2 Nickel(II) nitride Cu3N2 Copper(II) nitride Oxidation state/Charge ion → Li1+ O2- Formula compound Li2 O1 Video on polyatomic ions Writing Chemical Formula Step 1 : Write Oxidation state/charge Step 2 : Balance it, (electrically neutral) by cross multiply – as subscript
- 2. Polyatomicions Group of non-metals bonded together Oxidation state Oxidation state Oxidation state -1/+1 -2 -3 (OH)-1 Hydroxide (SO4)2- Sulphate (PO4)3- Phosphate (CN)-1 Cyanide (SO3)2- Sulphite (SCN)-1 Thiocyanate (CO3)2- Carbonate (NO3)-1 Nitrate (S2O3)2- Thiosulphate (NO2)-1 Nitrite (Cr2O7)2- Dichromate (NH4)+1 Ammonium Polyatomic ions Li2(CO3) Mg(CO3) Al2(CO3)3 Fe(CO3) Ni(CO3) Cu(CO3) Li(OH) Mg(OH)2 Al(OH)3 Fe(OH)2 Ni(OH)2 Cu(OH)2 Li2(SO4) Mg(SO4) Al2(SO4)3 FeSO4 Ni(SO4) Cu(SO4) Video on polyatomic ions Ionic Compound Writing Chemical Formula Metal/Cations/+ve ions Cations/Metals/+ve ions Gp1 Gp 2 Gp3 Transition metals ions ( variable oxidation states) Oxidation state +1 Oxidation state +2 Oxidation state +3 Sc +3 Ti +2 +3 V +2 +3 Cr +2 +3 +6 Mn +2 +3 +6 +7 Fe +2 +3 Co +2 +3 Ni +2 Cu +1 +2 Li 1+ Be2+ Sc 3+ Ti 2+ Ti 3+ V 2+ V 3+ Cr 2+ C r3+ Cr6+ Mn 2+ Mn 3+ Mn 6+ Mn 7+ Fe 2+ Fe 3+ Co2+ Co3+ Ni2+ Cu1+ Cu2+ Na 1+ Mg2+ Al 3+ K 1+ Ca2+ Oxidation state/Charge ion → Li1+ (CO3)2- Formula compound Li2 (CO3 )1 Step 1 : Write Oxidation state/charge ion Step 2 : Balance it, (electrically neutral) by cross multiply – as subscript
- 3. Redox (Oxidation and Reduction) Oxidation – Gain of oxygen ↑ Oxidation – Loss of hydrogen ↓ Reduction – Gain of hydrogen ↑ Reduction – Loss of oxygen ↓ Oxidation Reduction Gain oxygen ↑ Loss oxygen ↓ Gain oxidation Number ↑ Loss oxidation Number ↓ Loss hydrogen↓ Gain hydrogen ↑ Loss electron ↓ Gain electron ↑ Ca + O2 → CaO CH4 + 2O2 → CO2+ 2H2O gain oxygen gain oxygen Zn + CuO → ZnO + Cu PbO + CO → Pb + CO2 loss oxygen loss oxygen H2S + CI2 → S +2HCI loss hydrogen H2S + CI2 → S + 2HCI Redox- Oxidation state change - Electron transfer CH4 + 2O2 → CO2 + 2H2O gain hydrogen Zn + CuSO4 → ZnSO4 + Cu Zn + CI2 → ZnCI2 No gain/loss oxygen/hydrogen Redox gain oxygen gain hydrogen Reduction Oxidation Are these redox rxns?
- 4. Redox- Oxidation state change - Electron transfer Zn + CuSO4 → ZnSO4 + Cu Zn + CI2 → ZnCI2No gain/loss oxygen/hydrogen Are these redox rxns? Yes – change in oxidation number Yes – loss/gain of electron Yes – change in oxidation number Yes – loss/gain of electron✓ ✓ • Assuming bond are ionic with diff EN values (unless bet same element) • Assign each atom, measure of electron control relative to atom in pure element • Apparent/imaginary charge it has when bonded to diff elements • Unequal sharing electron based on electronegativity • + means lose electron • - means gain electron Oxidation State/Number/ON Rules Oxidation Reduction Gain oxygen ↑ Loss oxygen ↓ Gain oxidation Number ↑ Loss oxidation Number ↓ Loss hydrogen↓ Gain hydrogen ↑ Loss electron ↓ Gain electron ↑ H CI xx xx • x ∂-∂+ +1 -1Oxidation number Oxidation state (sign, number) +2 NOT 2+ Oxidation state and formal charge useful tool for electron book- keeping. They are not REAL! - Oxidation state -Assume bond ionic with diff EN values (unless bet same element) - Formal charge – Assume bond covalent Redox (Oxidation and Reduction)
- 5. Redox (Oxidation and Reduction) • Assuming bond are ionic with diffEN values (unless bet same element) • Assign each atom, measure of electron control relative to atom in pure element • Apparent/imaginary charge it has when bonded to diff elements • Unequal sharing electron based on electronegativity • + means lose electron • - means gain electron Oxidation Number/ON Rules H CI xx xx • x ∂-∂+ +1 -1Oxidation number Oxidation state (sign, number) +2 NOT 2+ Oxidation state and formal charge useful tool for electron book- keeping. They are not REAL! - Oxidation state -Assume bond ionic with diff EN values (unless bet same element) - Formal charge – Assume bond covalent xx CI CI Oxidation Number/ON Rules H CI Na CI Imagine electron move to more EN element O C O oo o o xxoo oo xx xx o x Equal sharing xx xx xx o x +1 -1 Unequal sharing 0 0 xx xx xx o x +1 -1 Complete transfer ox ox o o oo oo oo oo o o xo xo Unequal sharing -2 +4 -2 H O H CI2 H CI Na CI C O2 +1 -1 -1+1 +4 -2 oo o x o x +1 -2 +1 Unequal sharing H N H H o x o x o x oo +1 -3 +1 +1 Unequal sharing H2 O N H3 H C H H H o x o xo x o x +1 +1 +1 +1 -4 C H4 +1 -2 -3 +1 -4 +1 CI C CI CI CI -1 -1 -1 -1 xx xx xx xx xx xx xx xx xx xx xx xx xo xo ox ox +4 C CI4 +4 -1 0
- 6. Exceptions Element ON Exception Example Hydrogen +1 H +1 -1 Bond to metal Metal hydride NaH CaH2 Oxidation Number/States/ON Rules Imagine electron move to more EN element • Assuming bond are ionic with diff EN values (unless bet same element) • Assign each atom, measure of electron control relative to atom in pure element • Apparent/imaginary charge it has when bonded to diff elements • Unequal sharing electron based on electronegativity H CI xx ∂- xx • x Oxidation number +1 -1 ∂+ xx H CI xx xx xx +1 -1 o x Unequal sharing H CI +1 -1 Na Ho x +1 -1 Complete transfer Na H +1 -1 Exceptions Element ON Exception Example Oxygen -2 O -2 +2 Bond to fluorine F2O Exceptions Element ON Exception Example Oxygen -2 O -2 -1 Peroxide (O-O) H2O2 H O H F O F oo oo o x o x +1 -2 +1 Unequal sharing H2 O +1 -2 oo oo xx xx xx xx xx xx ox ox -1 +2 -1 Unequal sharing F2 O -1 +2 O O HH O-O single bond oo oo oo oo ox ox o o equal sharing +1 -1 -1 +1 Unequal sharing H2 O2 +1 -1 EN fluorine higher ↑EN oxygen higher ↑ Oxidation Number/ON Rules Imagine electron move to more EN element O O H O H Oxidation state O different – depend element bond with – different EN values ! F O F O O xx xx xx xx xx xx Equal sharing 0 0 O2 0 oo oo oo oo ox ox +1 -2 +1 Unequal sharing H2 O +1 -2 xx xx xx xx xxxx ox ox -1 +2 -1 F2 O -1 +2 H H oo oo oo oo o o ox ox +1 -1 -1 +1 H2 O2 +1 -1 Unequal sharing Unequal sharing
- 7. Oxidation Number/ON Rules Imagine electron move to more EN element • Assuming bond are ionic with diff EN values (unless bet same element) • Assign each atom, measure of electron control relative to atom in pure element • Apparent/imaginary charge it has when bonded to diff elements • Unequal sharing electron based on electronegativity H CI xx ∂- xx • x Oxidation number +1 -1 ∂+ xx Oxidation Number/ON Rules Imagine electron move to more EN element O O H O H Oxidation state O different – depend element bond with – different EN values ! F O F O O xx xx xx xx xx xx Equal sharing 0 0 O2 0 oo oo oo oo ox ox +1 -2 +1 Unequal sharing H2 O +1 -2 xx xx xx xx xxxx ox ox -1 +2 -1 F2 O -1 +2 H H oo oo oo oo o o ox ox +1 -1 -1 +1 H2 O2 +1 -1 Unequal sharing Unequal sharing Oxidation Number/ON Rules Imagine electron move to more EN element Oxidation state N different – depend element bond with – different EN values ! H N H H H H O N O H O N O N O N O O O O ox ox o x x x x x +1 -3 +1 +1 -2 [N H4 ] -3 +1 +1 Unequal sharing xx ox ox xo xo xx oo oo oo oo +1 -2 +3 -2 Unequal sharing H N O2 +1 +3 -2 O ox ox xo xo xx oo o o o o +1 -2 +5 -2 -2 Unequal sharing H N O3 +1 +5 -2 ox ox xx oo xx oo o o o o o o o o o o o o o o o o oo oo -2 -2 -2 -5 -5-2 N2 O5 Unequal sharing -5 +2 +1 +
- 8. Oxidation Number/ON Rules Atoms uncombined free element state = ON = 0 Ion form – ON same as charged on ion 1 2 Mg Mg2+ Na Na+ O2 S8 O2- 3 ON for element same as its most common ion/group ON metal from Gp 1 – 3 ON non metal Gp 5 - 7 Anion/Nonmetal Gp 5 Gp 6 Gp 7 Oxidation state Oxidation state Oxidation state - 3 - 2 - 1 N 3- O 2- F 1- P 3- S 2- CI 1- Cation/Metal Gp 1 Gp 2 Gp 3 Oxidation state Oxidation state Oxidation state +1 +2 +3 Na 1+ Mg 2+ Al 3+ K1+ Ca 2+ Ga 3+ 4 CI2 0 0 0 0 0 +1 +2 -2 -1 -2 CI- S2- ON for transition metal varies Transition metal ions Transition metals ions (variable oxidation states) Sc +3 Ti +2 +3 V +2 +3 Cr +2 +3 +6 Mn +2 +3 +6 +7 Fe +2 +3 Co +2 +3 Ni +2 Cu +1 +2 Zn +2 Sc 3+ Ti 2+ Ti 3+ V 2+ V 3+ Cr 2+ Cr 3+ Cr 6+ Mn 2+ Mn 3+ Mn 6+ Mn 7+ Fe 2+ Fe 3+ Co 2+ Co 3+ Ni 2+ Cu 1+ Cu 2+ Zn 2+ Oxidation number Diff ON Charge on ion Click here on oxidation rulesClick here view simple step Notes Sc3+ Charge on Sc Oxidation number +3 Oxidation state -+ 3+
- 9. ON all atoms in polyatomic ion add up to charge of polyatomic ion ON all atoms in neutral molecule add up to 0 CO3 2- SO4 2- H2SO4 CO2 5 Oxidation Number/ON Rules HNO3 (+1)2 + (+6) + (-2)4 = 0 +1 +6 -2 (+4) + (-2)2 = 0 (+1) + (+5) + (-2)3 = 0 +4 -2 +1 +5 -2 (+4) + (-2)3 = -2 +4 -2 (+6) + (-2)4 = -2 NO3 1- +6 -2 (+5) + (-2)3 = -1 +5 -2 7 ON atom/molecule of element = 0 (NOT combined) H2 CI2 O2 Fe Cu Mg 0 0 0 0 0 0 8 Monoatomic ion – ON same as charged on ion Ionic compound Charge ion Oxidation number MgF2 Mg 2+ F 1- Mg (+2) F (-1) NaCI Na 1+ CI 1- Na (+1) CI (-1) KBr K 1+ Br 1- K (+1) Br (-1) CaI2 Ca 2+ I 1- Ca (+2) I (-1) Li3N Li 1+ N 3- Li (+1) N (-3) Al2O3 Al 3+ O 2- AI (+3) O (-2) 9 Formula compound Charge Oxidation number Name using oxidation number FeO Fe 2+ or 2+ +2 Iron (II) oxide Fe2O3 Fe 3+ or 3+ +3 Iron (III) oxide Cu2O Cu 1+ or 1+ +1 Copper (I) oxide CuO Cu 2+ or 2+ +2 Copper (II) oxide MnO2 Mn 4+ or 4+ +4 Manganese (IV) oxide MnO4 - Mn 7+ or 7+ +7 Manganese (VII) oxide K2Cr2O7 Cr 6+ or 6+ +6 Potassium dichromate (VI) Cr2O3 Cr 3+ or 3+ +3 Chromium (III) oxide Click here view chemguide notes 6
- 10. Oxidation Number/ON Rules 9 Metal more than one oxidation states, Roman numeral used Manganese Chromium Ionic compound MnSO4 MnO2 K2MnO4 KMnO4 K2Cr2O7 Cr2O3 Oxidation Number (+2) + (+6) + (-2)4 = 0 Mn (+2) (+4) + (-2)2 = 0 Mn (+4) (+1)2 + (+6) + (-2)4 = 0 Mn (+6) (+1) + (+7) + (-2)4 = 0 Mn (+7) (+1)2 + (+6)2 + (-2)7 = 0 Cr (VI) (+3)2 + (-2)3 = 0 Cr (III) IUPAC name Manganese (II) sulphate Manganese (IV) oxide Manganese (VI) Manganese (VII) Chromium (VI) Chromium (III) Iron Copper Ionic compound FeCI2 FeCI3 CuCI CuCI2 Oxidation Number (+2) + (-1)2 = 0 Fe (+2) (+3) + (-1)3 = 0 Fe (+3) (+1) + (-1) = 0 Cu (+1) (+2) + (-1)2 = 0 Cu (+2) IUPAC name Iron (II) chloride Iron (III) chloride Copper (I) chloride Copper (II) chloride Vanadium VO2 + VO 2+ (+5) + (-2)2 = +1 V (+5) (+4) + (-2) = +2 V (+4) Vanadium (V) Vanadium (IV) ON for underlined element in ionic compound10 Na2SO3 Na2SO4 NaNO2 NaNO3 (SO3)2- (SO4)2- (+1)2 + (+4) + (-2)3 = 0 (+1)2 + (+6) + (-2)4 = 0 (+1)+ (+3) + (-2)2 = 0 (+1)+ (+5) + (-2)3 = 0 (+4) + (-2)3 = -2 (+6) + (-2)4 = -2 ON for S = +4 ON for S = +6 ON for N = +3 ON for N = +5 ON for S = +4 ON for S = +6 +1 +4 -2 +1 +6 -2 +1 +3 -2 +1 +5 -2 +4 -2 +6 -2
- 11. Oxidation Number/ON Rules 11 ON for underlined element in compound OH-1 PO4 3- S2O3 2- CN-1 OCI-1 H2O2 (HCO3)-1 (-2) + (+1) = -1 (+5) + (-2)4 = -3 (+2)2 + (-2)3 = -2 (+4) + (-5) = -1 (-2) + (+1) = -1 (+1)2 + (-1)2 = 0 ON for O = -2 ON for P = +5 ON for S = +2 ON for C = +4 ON for O = -2 ON for O = -1 ON for C = +4 (+1) + (+4) + (-2)3 = -1 -2 +1 +5 -2 +2 -2 +4 -5 -2 +1 +1 -1 +1 +4 -2 Oxidation Reduction Gain oxygen ↑ Loss oxygen ↓ Loss hydrogen ↓ Gain hydrogen ↑ Redox (Oxidationand Reduction) Rxn involve gain/loss of oxygen/hydrogen CH4 + 2O2 → CO2 + 2H2O Gain hydrogen Oxygen reduction gain oxygen Carbon oxidation Rxn involve gain/loss of electron Oxidation Reduction Gain ON ↑ Loss ON ↓ Loss electron ↓ Gain electron ↑ - broader definition - cover more rxn types PbO + CO → Pb + CO2 Lead Reduction gain oxygen Carbon oxidation (-4) (+4) (0) (-2) ON ↑ ON ↓ oxygen reduced carbon oxidized PbO + CO → Pb + CO2 (+2) (0) lead reduced (+2) (+4) CH4 + 2O2 → CO2 + 2H2O ON ↑ carbon oxidized ON ↓ loss oxygen
- 12. Redox (Oxidation and Reduction) • Assuming bond are ionic with diff EN values (unless bet same element) • Assign each atom, measure of electron control relative to atom in pure element • Apparent/imaginary charge it has when bonded to diff elements • Unequal sharing electron based on electronegativity Oxidation Number/ON Rules H CI xx xx • x ∂-∂+ +1 -1Oxidation number Oxidation state (sign, number) +2 NOT 2+ Oxidation state useful tool for electron book- keeping. They are not REAL! - Oxidation state -Assume bond ionic with diff EN values (unless bet same element) xx Na CI OxidationNumber/ON Rules Imagine electron move to more EN element xx xx xx o x +1 -1 Complete transfer Na CI +1 -1 Ionic compound H CI Covalent compound xx xx +1 -1 xx o x H CI Unequal sharing +1 -1 Organic Covalent compound H C O H H H o x o x o X o X o x oo -2 +1 +1 +1 +1 -2 C H4 O Unequal sharing Still covalent bond Treat as imaginary ionic (loss/gain of electron control) Bonds are still covalent NOT ionic, keep track where electron going -2 +1 -2 (+1) + (-1) = 0 (+1) + (-1) = 0 (-2) + (+1)4 + (-2) = 0 O S O O oo xx oo xx oo xx -2 +6 -2 -2 S O3 +6 -2 (+6) + (-2)3 = 0Click here note ON for organic carbon oo oo oo o o o o o o
- 13. ON for carbon is NOT -2 Oxidation Number/ON Rules • Assuming bond are ionic with diff EN values (unless bet same element) • Apparent/imaginary charge it has when bonded to diff elements • Unequal sharing electron based on electronegativity - Oxidation state useful tool for electron book- keeping. They are not REAL! Oxidation state (sign, number) +2 NOT 2+ Click here here note ON carbon H C C H Oxidation state Carbon compound -4 CH4 -3 C2H6 -2 CH3CI -1 C2H2 0 CH2CI2 +1 CHCI2-CHCI2 +2 CHCI3 +3 C2CI6 +4 CCI4 ON carbon (Organic molecules) Average oxidation number bet carbon is taken! Different ON states Don’t need to take average ON bet carbons Oxidation state Carbon compound -3 and -1 CH3CH2OH -3 and +1 CH3CHO Different ON states o x o x +1 -1 -1 +1 C2 H2 -1 +1 ox ox ox Equal sharing (-1)2 + (+1)2 = 0 H C C O H H H o X o x o X o X oo o x o x H H o X oo o x +1 +1 -2 +1 +1 +1 +1-1-3 C2 H6 O1 x +1 -2 2x + 6 -2 = 0 x = -2 ON C in CH3 = -3 ON C in CH2 = -1 Average ON C = -2 H C C O H o x +1 H H o X o X o x o X oo xo xo oo +1 +1 +1 -3 -2 +1 C2 H4 O1 x +1 -2 2x + 4 -2 = 0 x = -1 ON for carbon is NOT -1 ON C in CH3 = -3 ON C in CH = +1 Average ON C = -1
- 14. ON C in CH3 = -3 ON C in COOH = +1 Average ON C = 0 +3 Oxidation Number/ON Rules • Assuming bond are ionic with different EN values (unless bet same element) • Apparent/imaginary charge it has when bonded to diff elements • Unequal sharing electron based on electronegativity - Oxidation state useful tool for electron book- keeping. They are not REAL! Oxidation state (sign, number) +2 NOT 2+ Click here here note ON carbon ON carbon (Organic molecules) Average oxidation number bet carbon is taken! Oxidation state carbon Carbon compound -3 and +3 CH3COOH H C C O H H H o X o x o X o X oo o x o x H H o X oo o x +1 +1 -2 +1 +1 +1 +1-1 C2 H6 O1 x +1 -2 2x + 6 -2 = 0 x = -2 ON C in CH3 = -3 ON C in CH2 = -1 Average ON C = -2 H C C O H o x +1 H H o X o X o x o X oo xo xo oo +1 +1 +1 -3 -2 +1 C2 H4 O1 x +1 -2 2x + 4 -2 = 0 x = -1 ON for carbon is NOT -2 ON for carbon is NOT -1 ON C in CH3 = -3 ON C in CH = +1 Average ON C = -1 H C C O H H O H +1 o x o X +1 +1 o X -3 -3 o x +1 oo oo oo oo xx o o o x o x -2 ON for carbon is NOT 0 C2 H4 O2 x +1 -2 2x + 4 -4 = 0 x = 0 Oxidation state carbon Carbon compound -3 and -1 CH3CH2OH Oxidation state carbon Carbon compound -3 and +1 CH3CHO
- 15. NOT Redox rxn • NO Loss/gain electron • NO change in oxidation number Concept Map Type of chemicalreaction Combustion/Respiration rxn Displacement rxn Synthesis rxn Decompositionrxn Single Displacement Double Displacement Acid/Base rxn Involve oxygen!! CH4 + 2O2→ CO2+ 2H2O C6H12O6 +6O2 → 6CO2+6H2O Redox rxn • Loss/gain electron • Change in oxidation number Zn + 2HCI→ H2+ ZnCI2 Zn + CuO → ZnO + Cu Redox rxn • Loss/gain electron • Change in oxidation number KI + Pb(NO3)2→ PbI2 + KNO3 Type of chemicalreaction HCI + NaOH→ NaCI + H2O MgO + 2HCI→ MgCI2 + H2O 2H2 + O2→ 2H2O 2KCIO3 → 2KCI + 3O2 Redox rxn • Loss/gain electron • Change in oxidation number Redox rxn • Loss/gain electron • Change in oxidation number NOT Redox rxn • NO Loss/gain electron • NO change in oxidation number H CI + Na O H → NaCI + H2 O +1 -1 +1 -2 +1 +1 -1 +1 -2 2H2 + O2 → 2H2O 0 0 +1 -2 2K CI O3 → 2K CI + 3O2 +1 +5 -2 +1 -1 0 C6 H12 O6 + 6O2 → 6C O2 + 6H2 O Zn + 2H CI → H2 + Zn CI2 0 +1 -1 0 +2 -1 K I + Pb (N O3)2 → Pb I2 + K N O3 +1 -1 +2 +5 -2 +2 -1 +1 +5 -20 +1 -2 0 +4 -2 +1 -2
- 16. Oxidation state Carbon compound -4 CH4 -3 C2H6 -2 CH3CI -1 C2H2 0 CH2CI2 +1 CHCI2-CHCI2 +2 CHCI3 +3 C2CI6 +4 CCI4 Different ON/ states for Carbon Different ON/ states for sulphur Carbon attach to H – ON lower ↓ - Carbon less oxidized - Carbon attract electron Carbon attach to CI/O (EN ↑) – ON higher ↑ - Carbon more oxidized - Carbon lose electron Oxidation state Sulphur compound -2 H4S 0 S8 +2 SCI3 +4 SO2 +4 SO3 2- +6 SO3 +6 H2SO4 Sulphur attach to H /CI – ON lower ↓ - Sulphur less oxidized - Sulphur attract electron Sulphur attach to O (EN ↑) – ON higher ↑ - Sulphur more oxidized - Sulphur lose electron Oxidation numbers and name Formula ion Charge Oxidation number Name using ON CrO4 2- 2- ON for Cr +6 Chromate (VI) Cr2O7 2- 2- ON for Cr +6 Dichromate (VI) MnO4 - 1- ON for Mn +7 Manganate (VII) MnO4 2- 2- ON for Mn +6 Manganate (VI) CIO - 1- ON for CI +1 Chlorate (I) CIO3 - 1- ON for CI +5 Chlorate (V) CIO2 - 1- ON for CI +3 Chlorate (III) CIO4 - 1- ON for CI +7 Chlorate (VII) Formula compound Charge Oxidation number Name using oxidation number FeO Fe 2+ or 2+ +2 Iron (II) oxide Fe2O3 Fe 3+ or 3+ +3 Iron (III) oxide Cu2O Cu 1+ or 1+ +1 Copper (I) oxide CuO Cu 2+ or 2+ +2 Copper (II) oxide MnO2 Mn 4+ or 4+ +4 Manganese (IV) oxide MnO4 - Mn 7+ or 7+ +7 Manganese (VII) oxide K2Cr2O7 Cr 6+ or 6+ +6 Potassium dichromate (VI) Cr2O3 Cr 3+ or 3+ +3 Chromium (III) oxide
- 17. Period 2 Shared electron cloud closer to O Electronegativity Electronegativity (EN) •Tendency of atom to attract/pull shared/bonding electron to itself •EN value higher – pull/attract electron higher (EN value from 0.7 – 4) EN highestEN lowest Factors affecting EN value •Size of atom/distance– small size/distance – stronger attraction for electron •Nuclear charge – higher nuclear charge – stronger attraction for electron Electronegativity •EN increase up a Group •EN increase across a Period F CI Br I Size increase Attraction electron decrease EN lower Size Be +4 Li +3 B +5 N +7 O +8 F +9 EN increase across period 2 Nuclear charge EN increase across period 2 Nuclear charge increase Strong attraction for electron EN increase Gp 17 C +6 EN decrease down gp 17
- 18. FORMAL CHARGE (FC) Tool/Model for comparing which Lewis structures is more acceptable Lewis structure SO2 Which is acceptable? Lewis structure SO3 Formal Charge •Treats covalentbond with equal electron distribution no EN diff bet atom •Electronegativeatom has negative while least electronegative atom has positive formal charge. Formula formal charge Click here video formal chargesClick here video formal charges V - valence electrons of atom L – Lone pair electron B - electrons shared in covalent bonds in the molecule ✓ ✓ All resonance structure contribute to electronic structure. Real structureis combination of them. Lowest formal charge (stable), contribute more than less stable structure. Sum of formal charges must be zero for neutral or equal to charge on ion. L + Formal charge concept Formal charge NOT real !!
