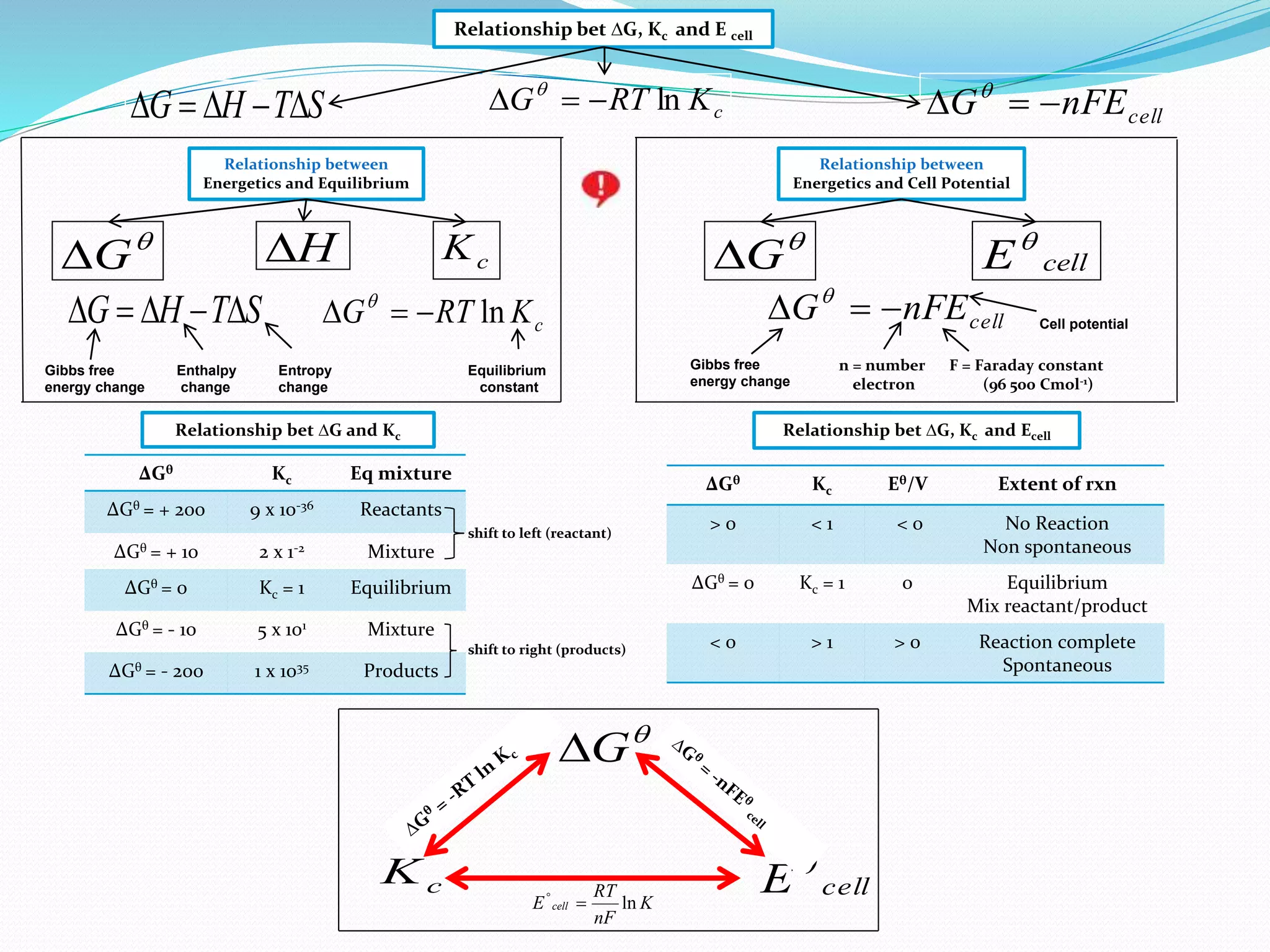
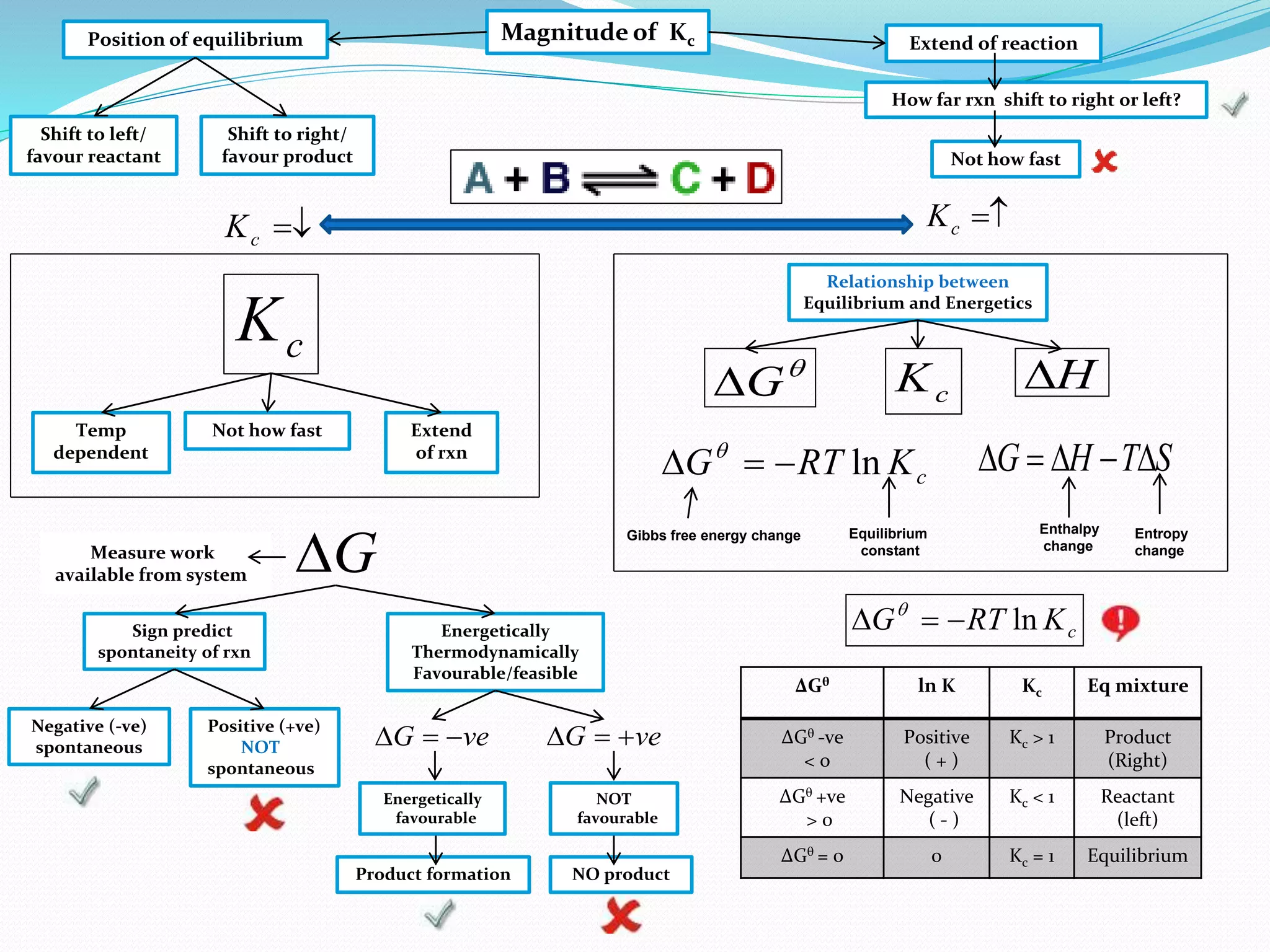
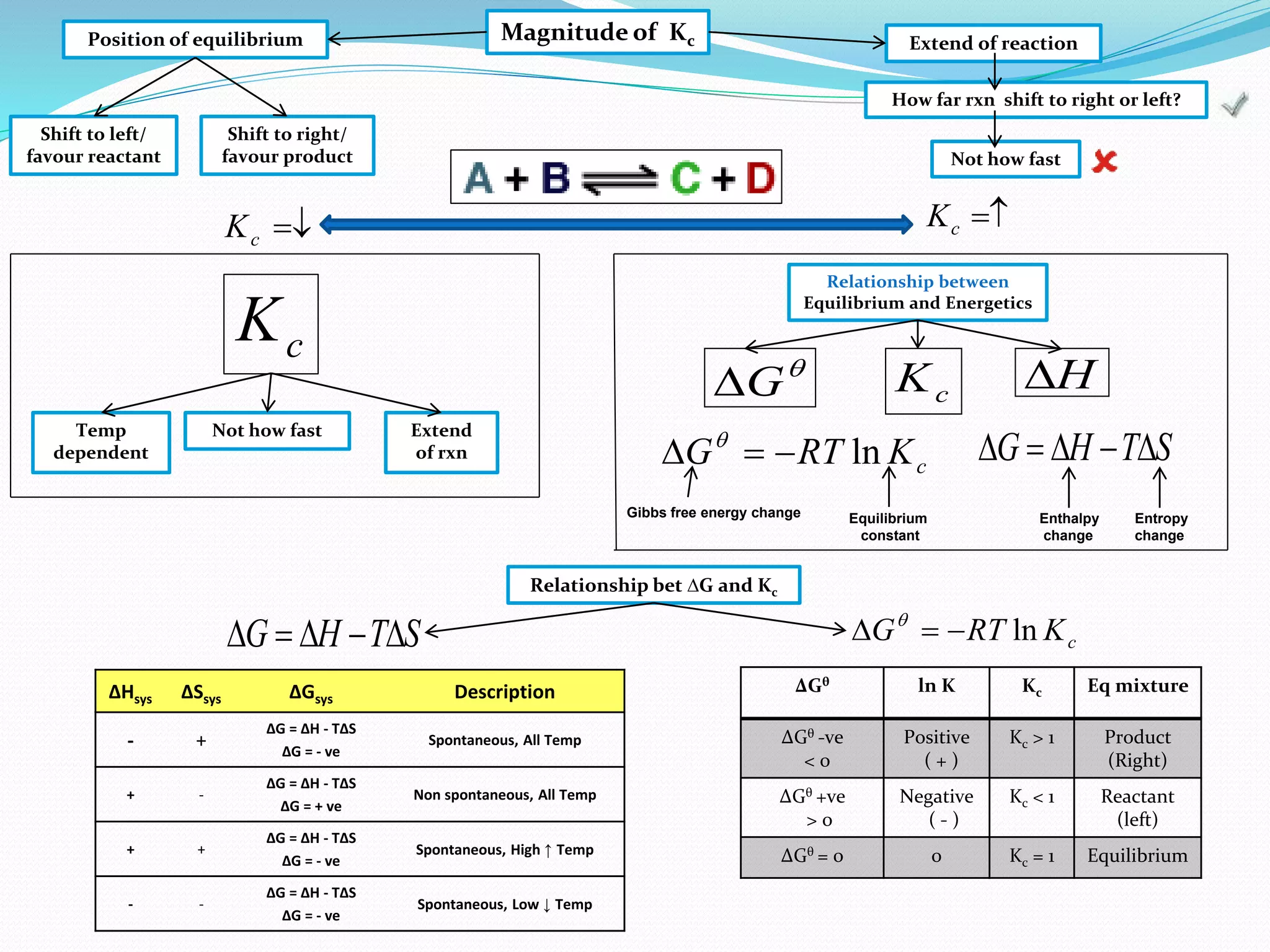
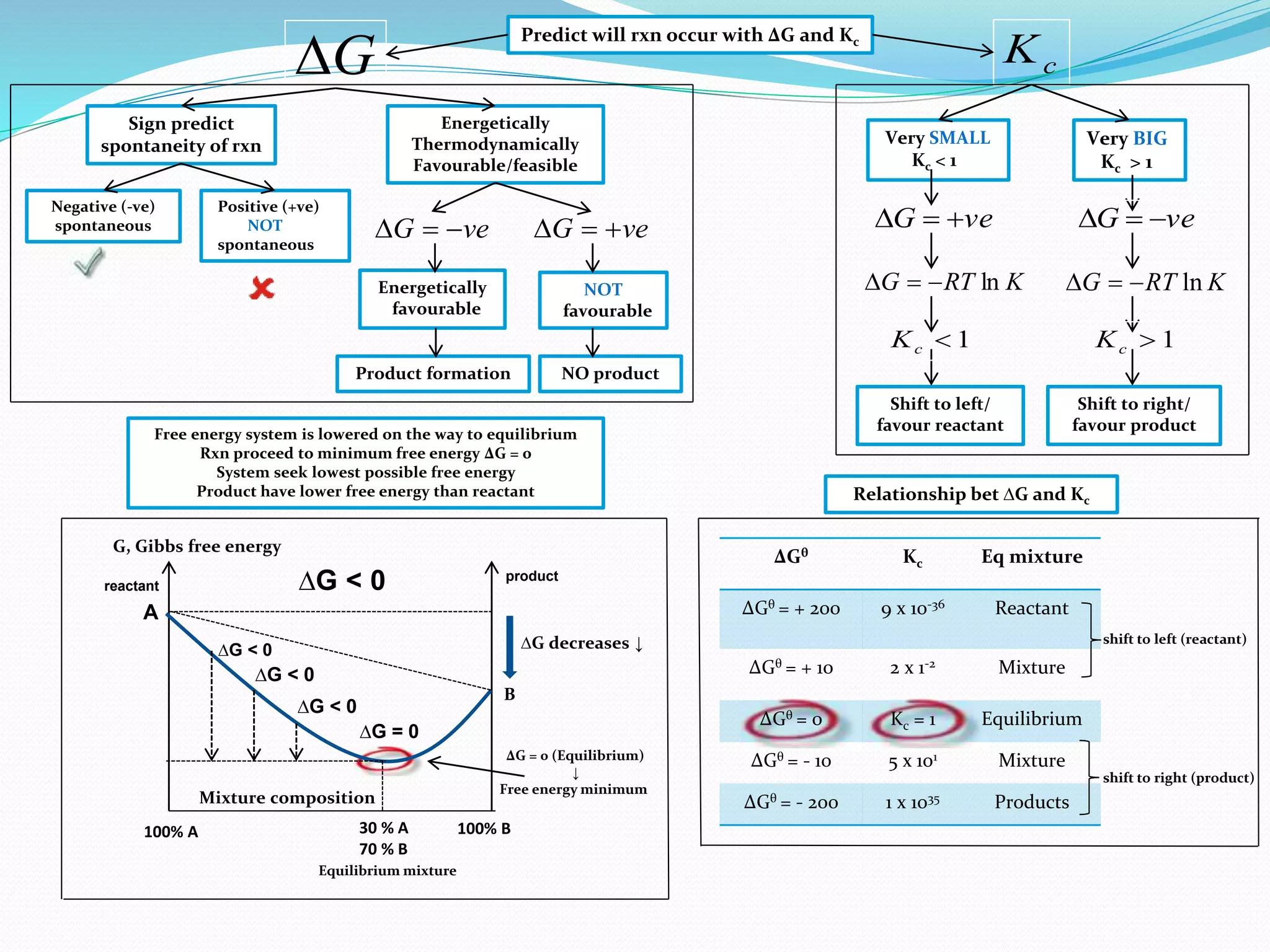
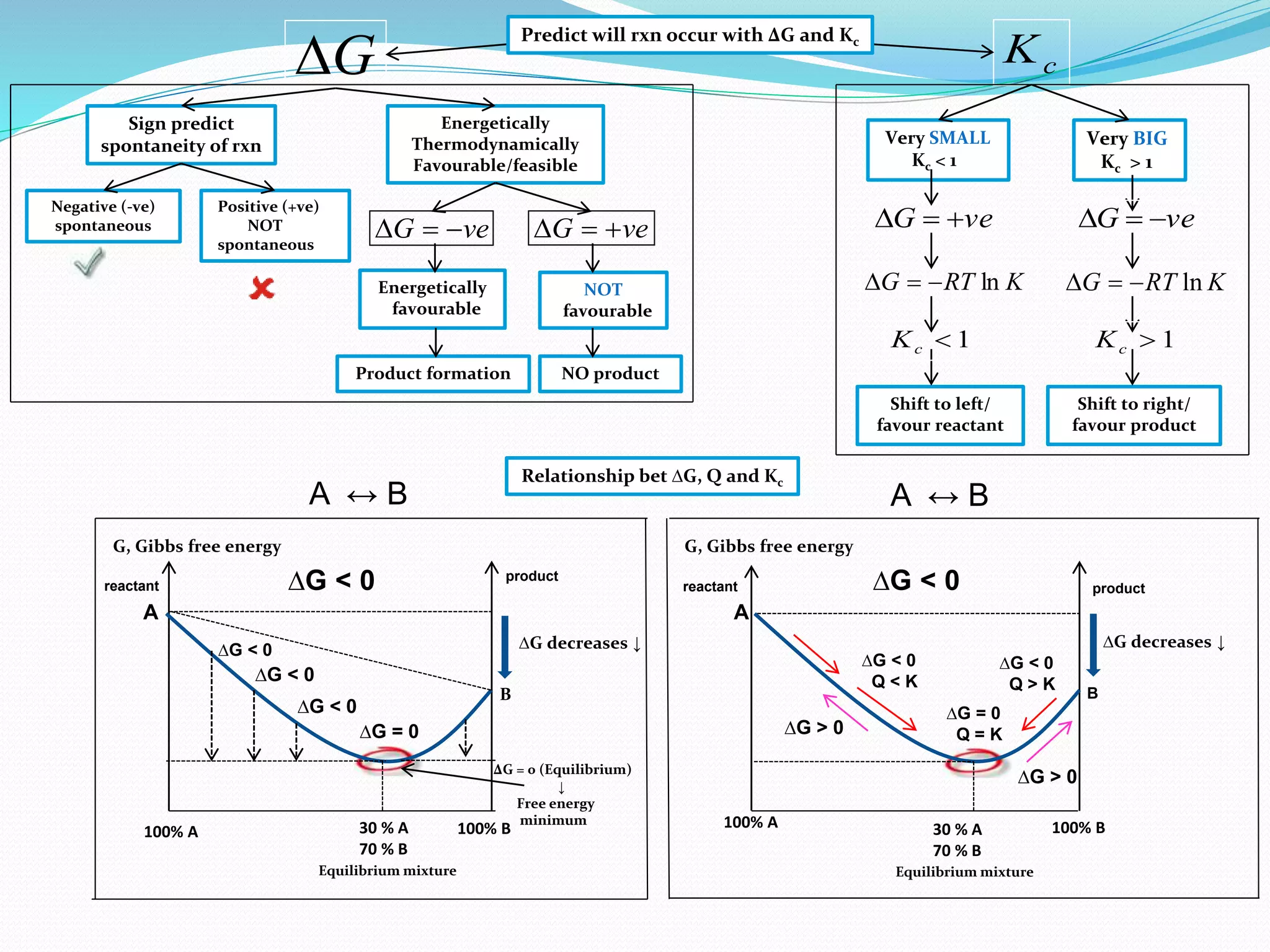
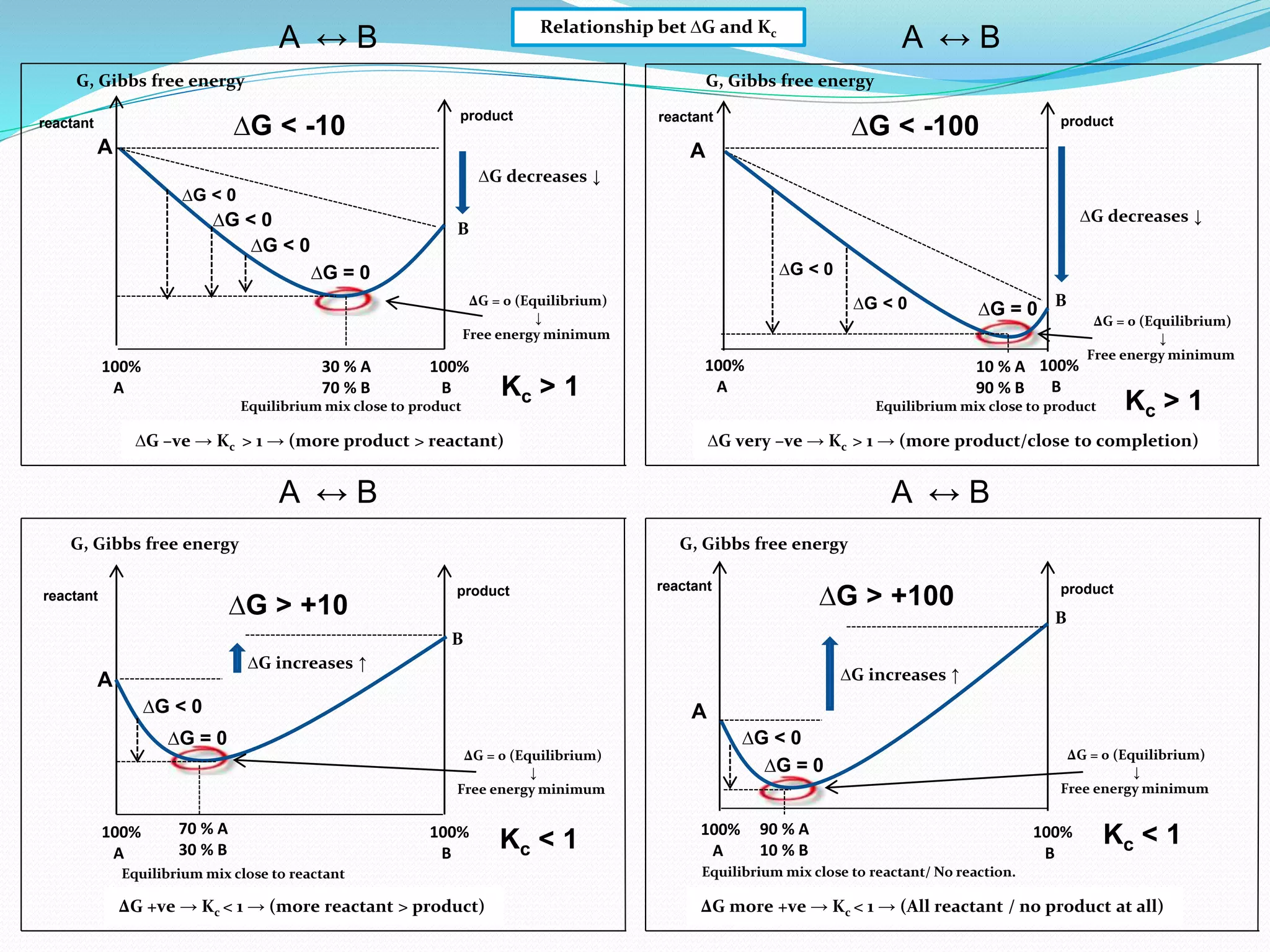
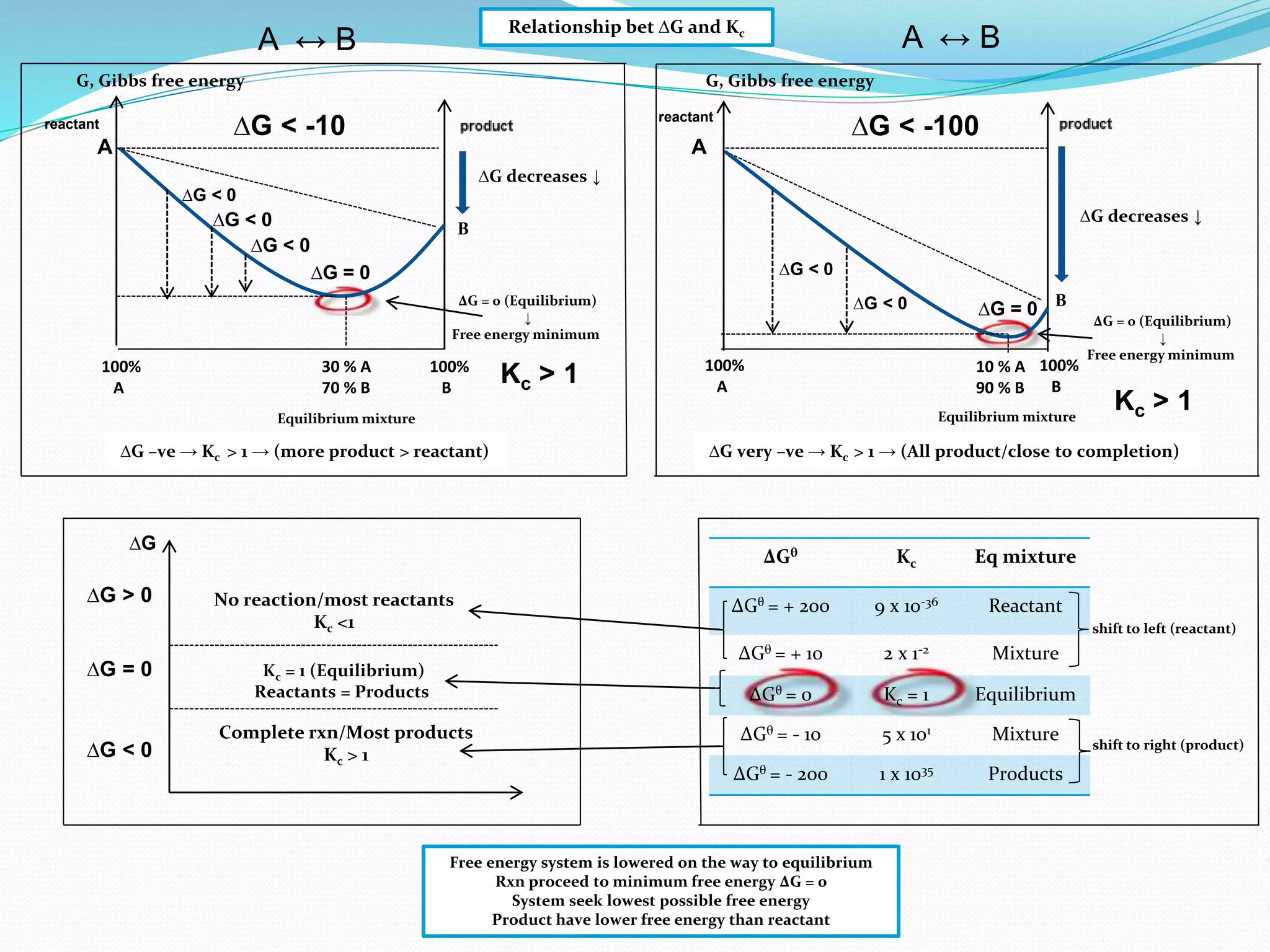
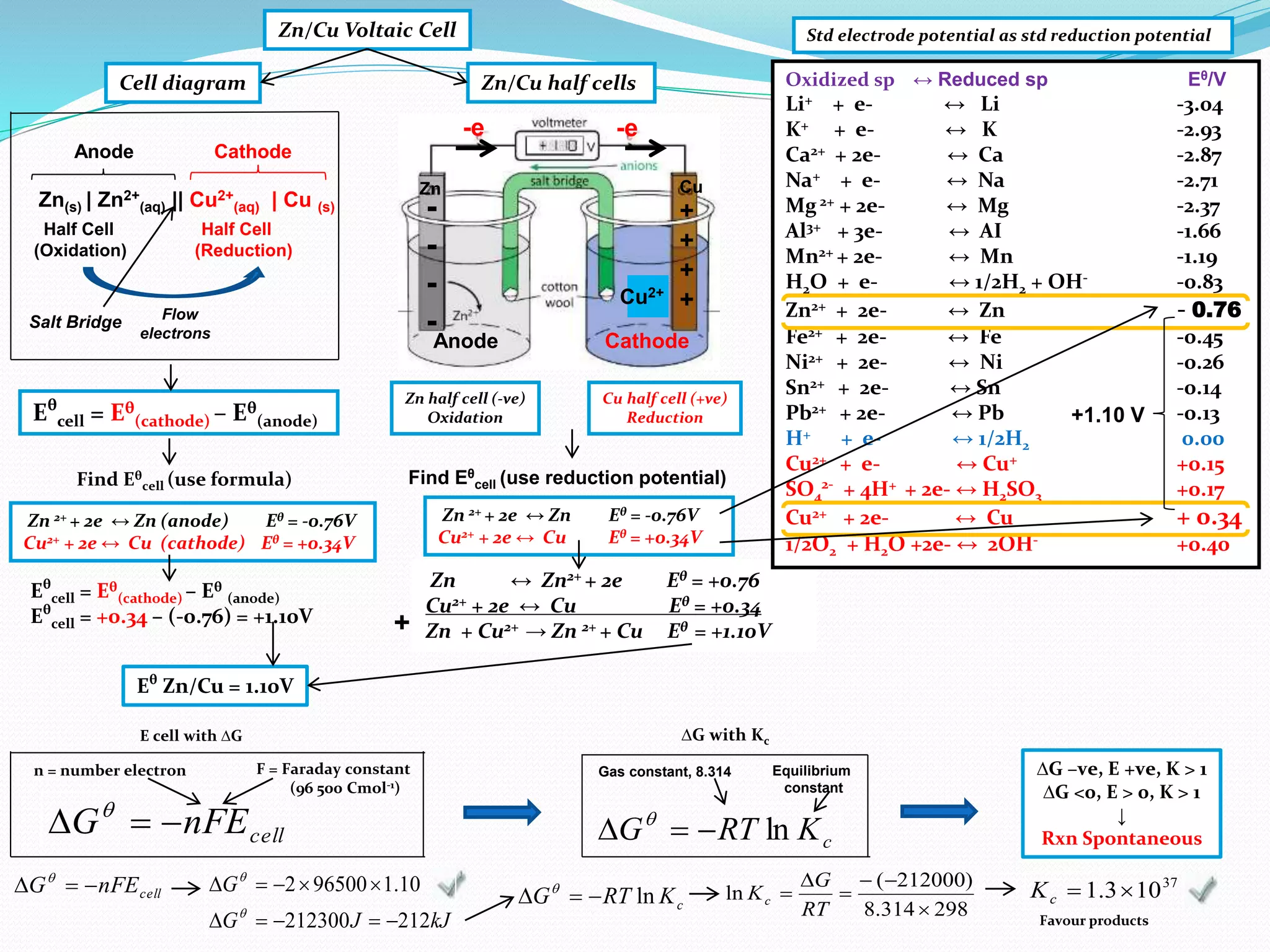
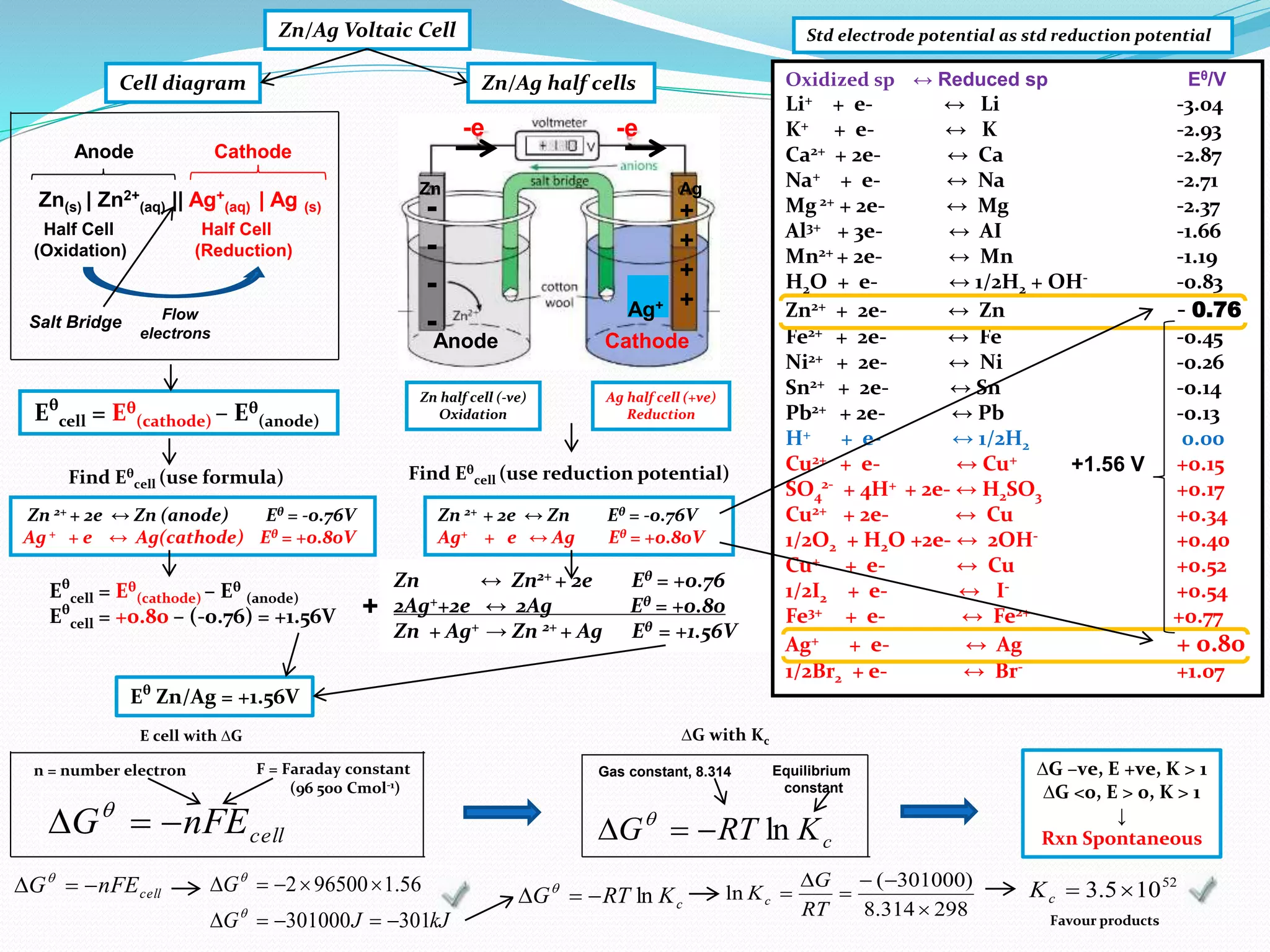
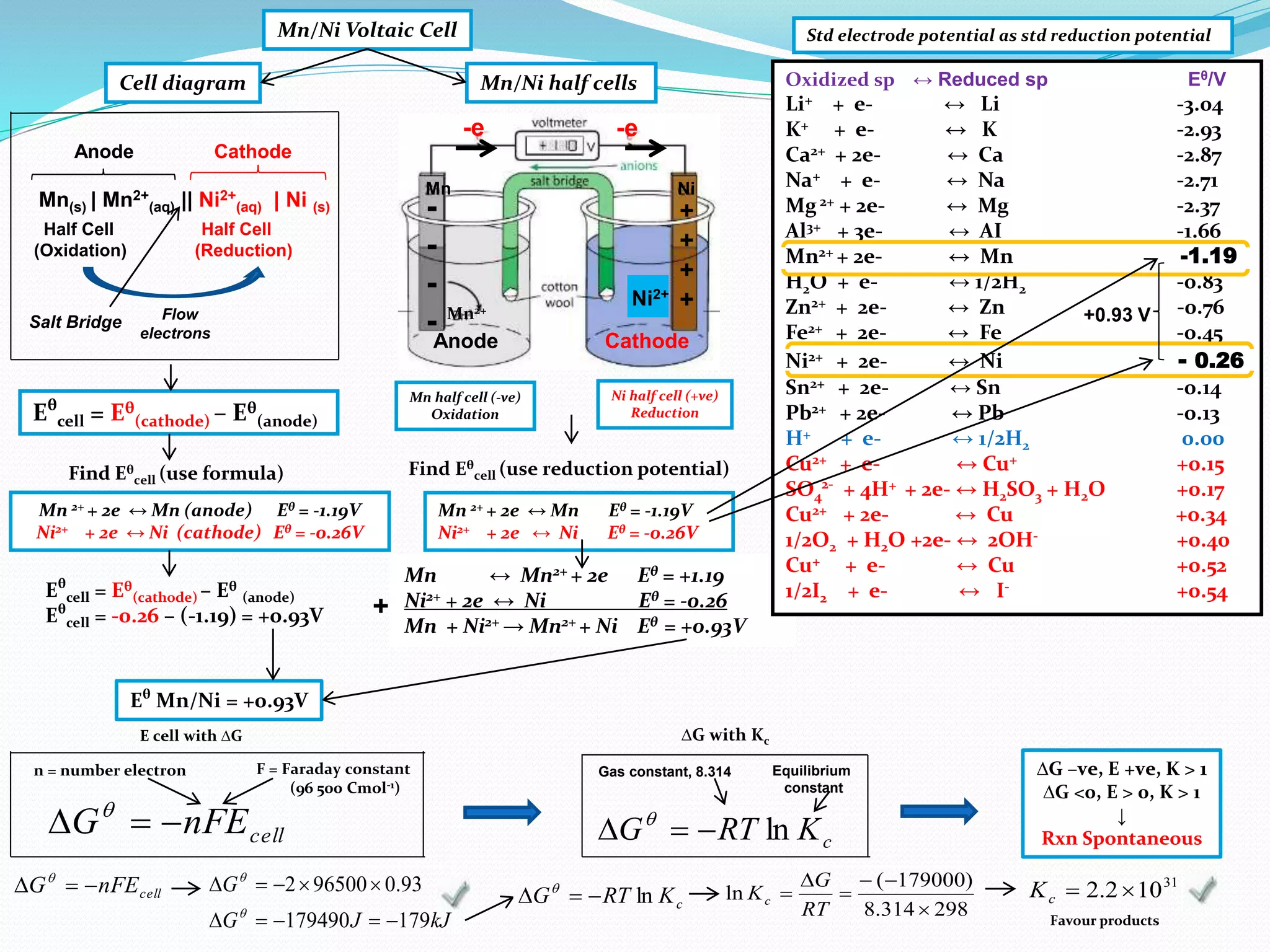
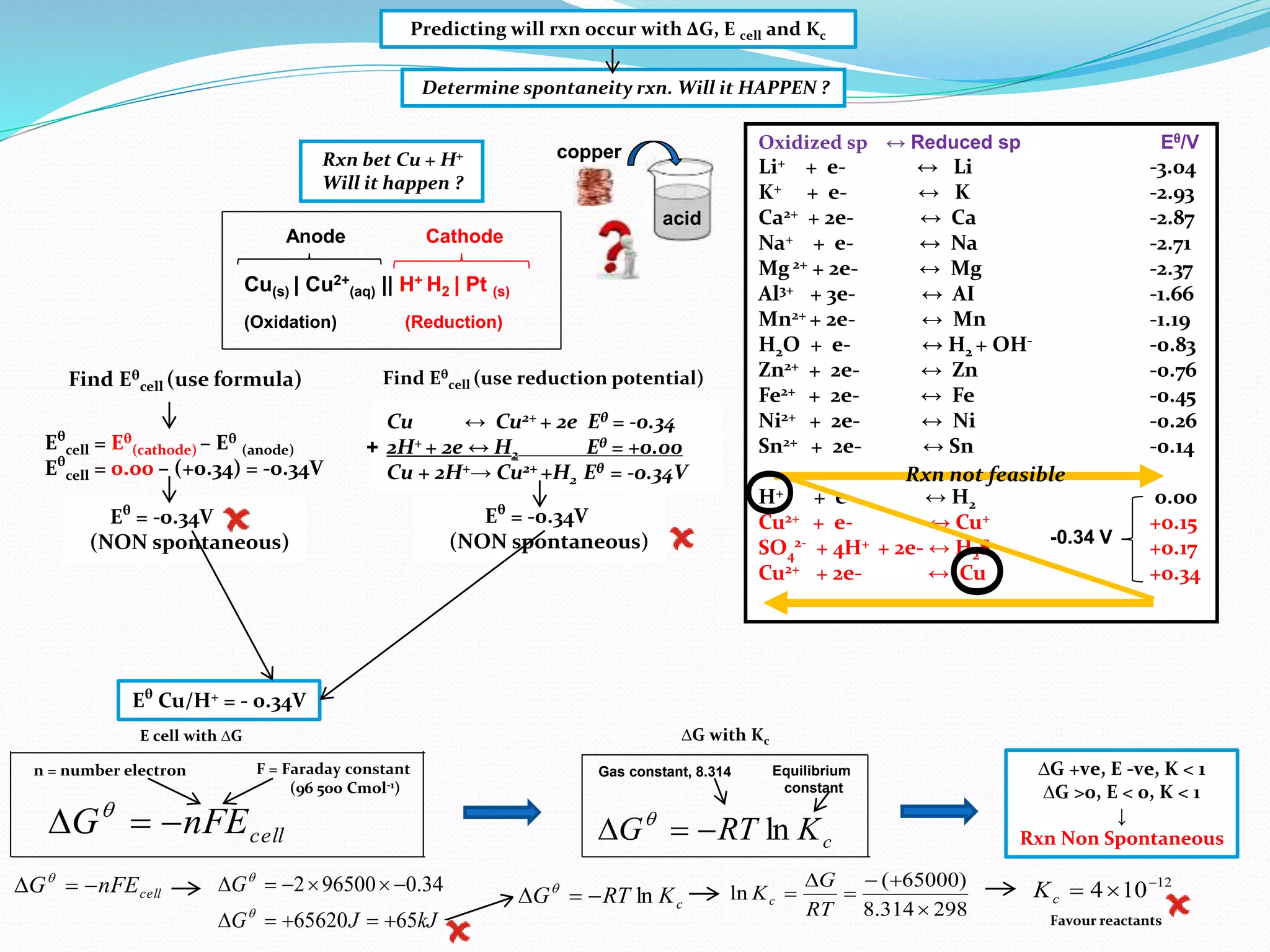
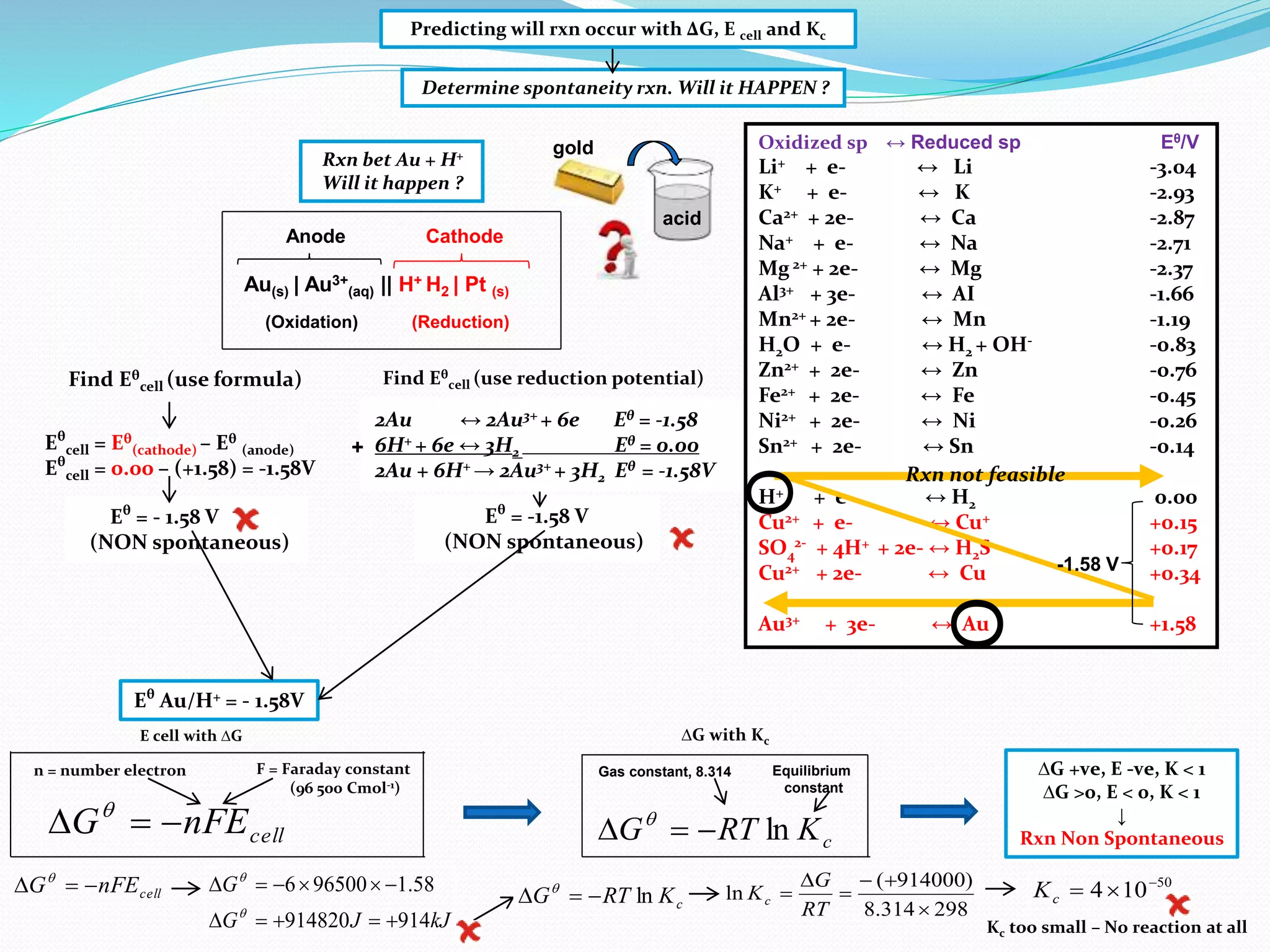
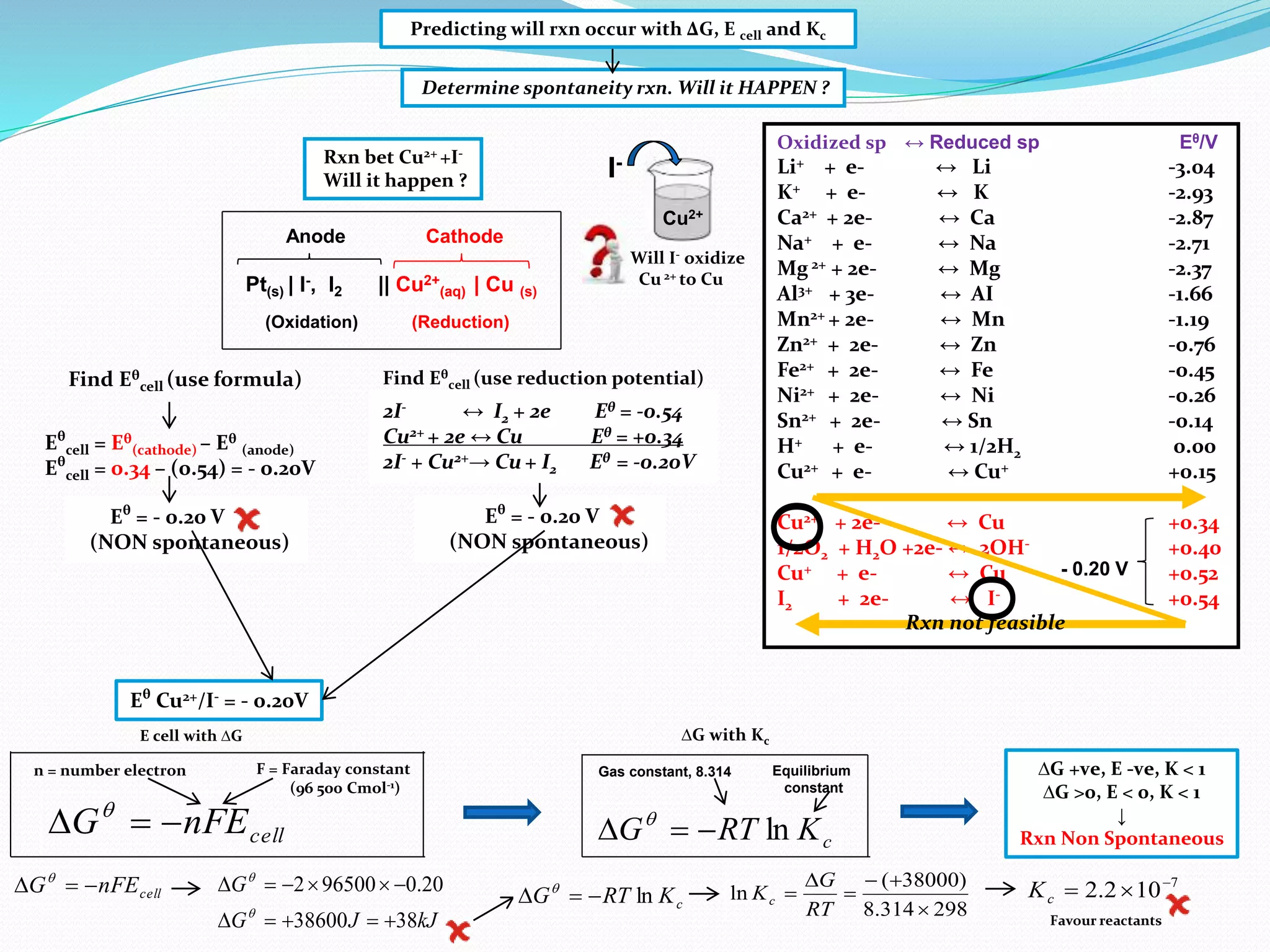
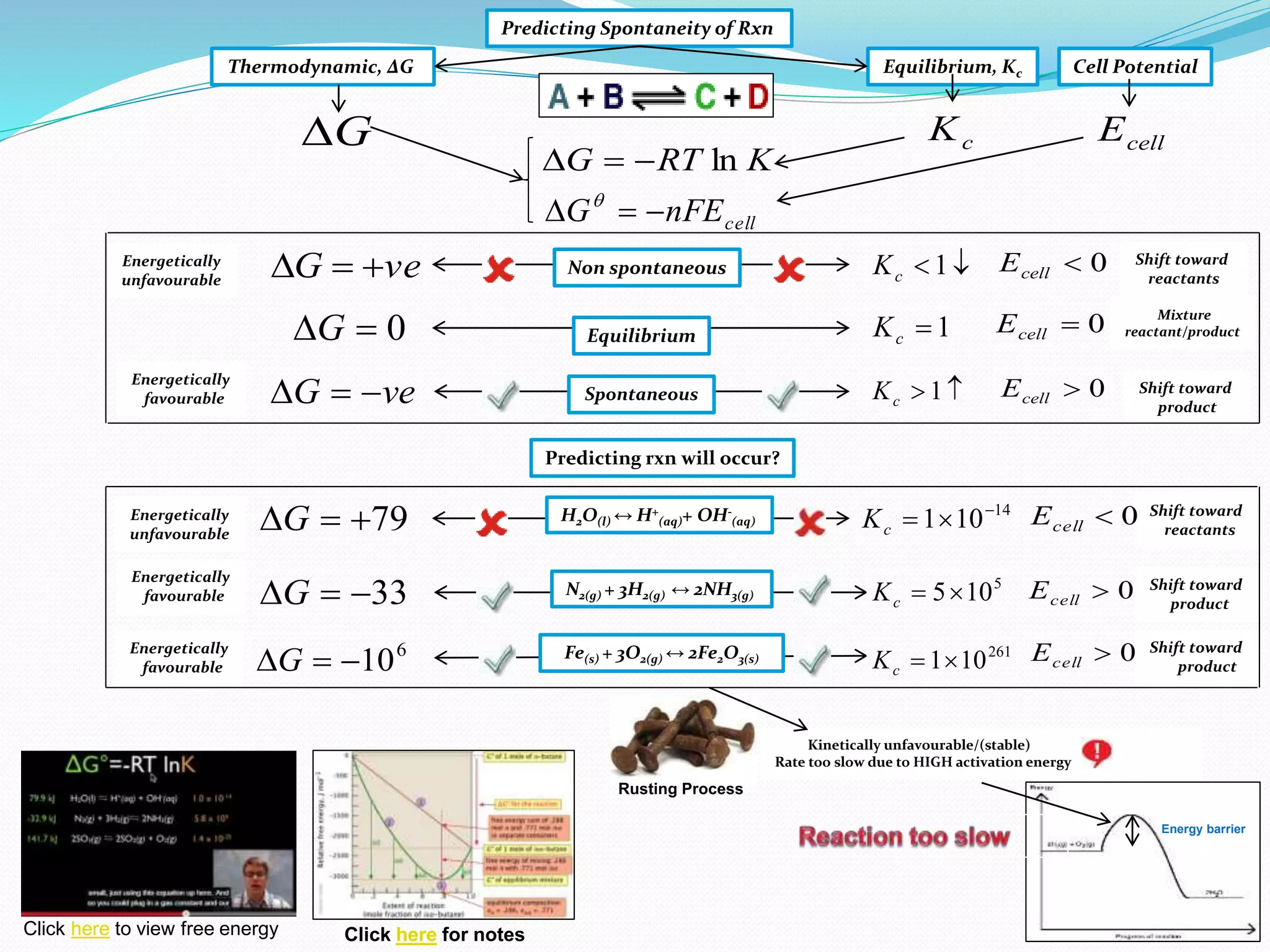
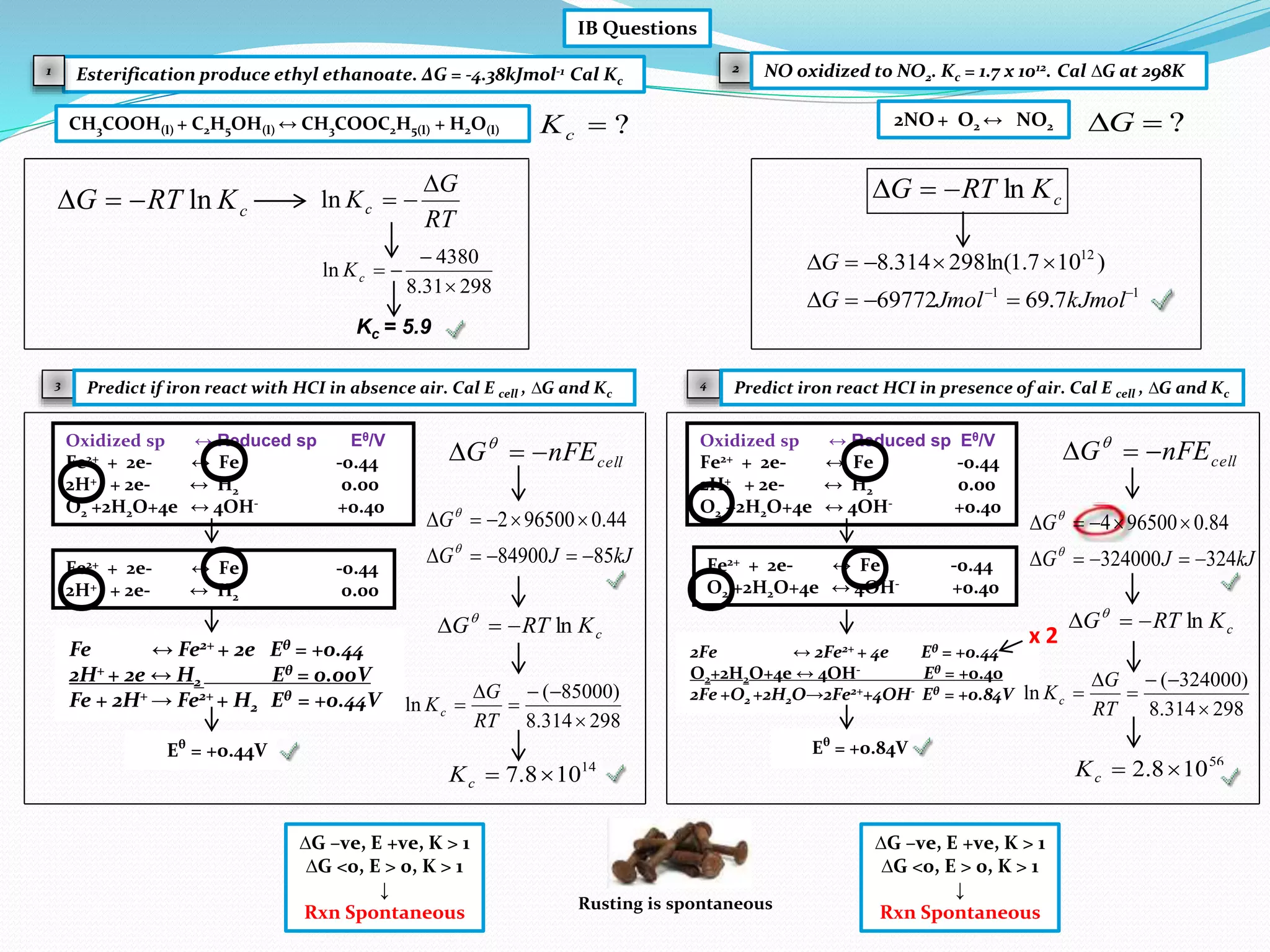
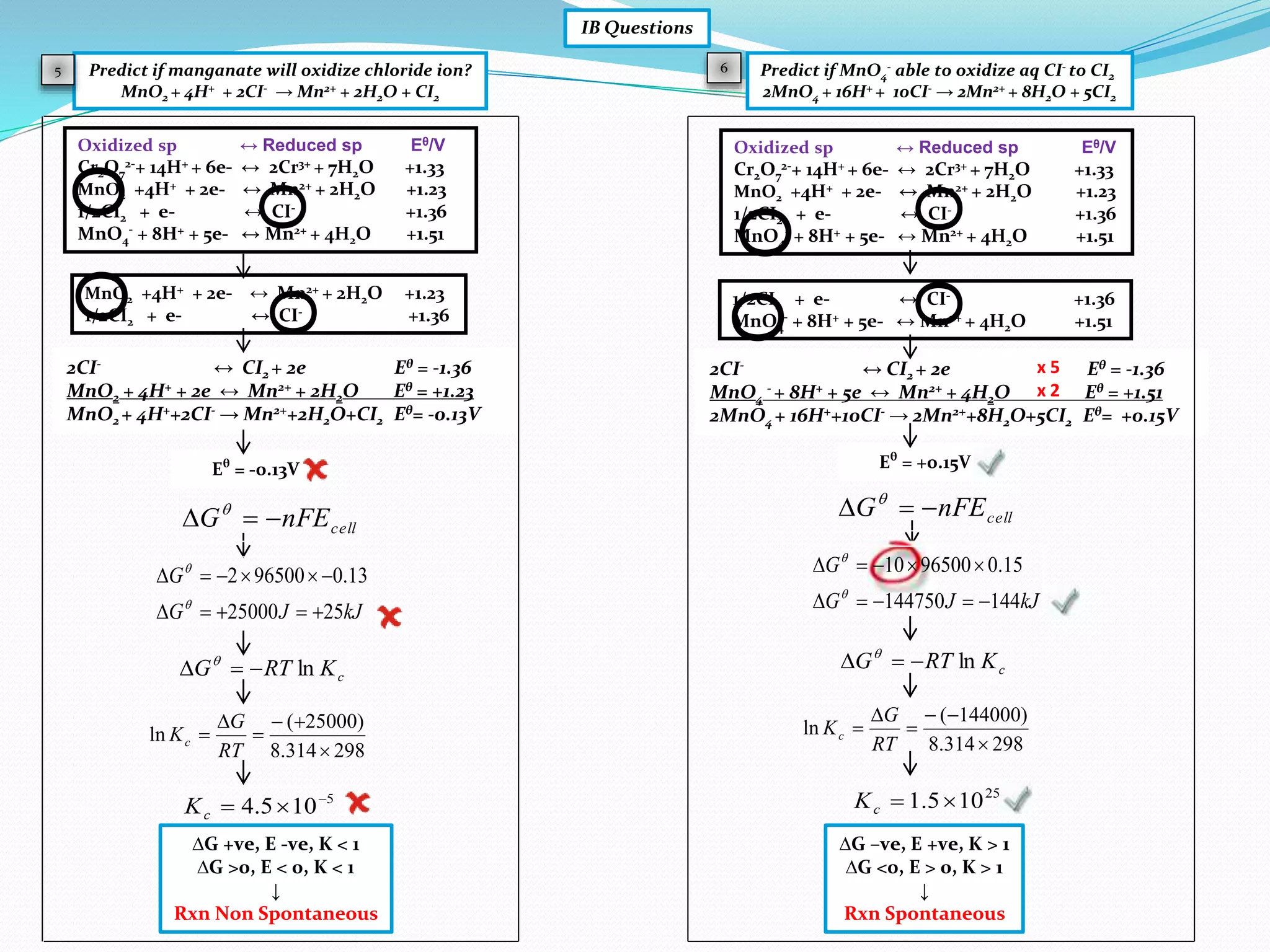
This document provides a tutorial on Gibbs free energy change, equilibrium, and cell potential in electrochemistry. It discusses the relationships between Gibbs free energy change (ΔG), entropy change (ΔS), enthalpy change (ΔH), equilibrium constant (Kc), and cell potential (Ecell). Graphs and equations are presented to show how ΔG relates to the position and extent of chemical equilibrium. The standard reduction potentials of various half-cell reactions are also listed.