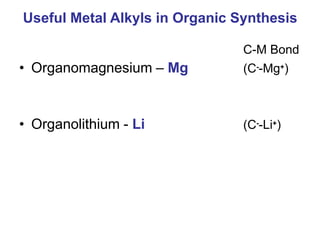
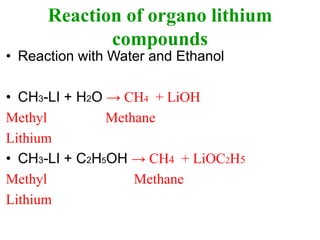
This document provides information about organometallic compounds, specifically discussing metal alkyls and their uses in organic synthesis. It describes how metal alkyls contain a metal-carbon bond and common metals include Li, Na, K, Mg, and transition metals. Grignard reagents and organolithium compounds are discussed as useful metal alkyls. Their reactions with compounds like formaldehyde, CO2, esters, epoxides, aldehydes, ketones, acetyl chloride, and cyanides are summarized. Specific examples of methylmagnesium bromide reactions are also provided.