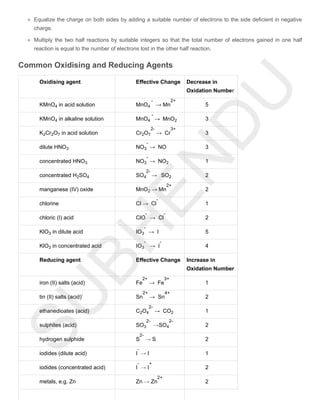
Electrochemistry deals with chemical changes caused by electric current. Substances can be conductors or non-conductors of electricity. Conductors include metals and electrolytes, which conduct by electron or ion movement respectively. Redox reactions involve the transfer of electrons from one species to another, changing their oxidation states. Balancing redox reactions uses half-reactions and ensures equal electron transfer.



![Both the electrodes can be fitted in the
same compartment
The electrodes are fitted in different
compartment
Standard electrode potential: The potential difference developed between metal electrode and the solution of its
ions of unit molarity (1M) at 25°C (298 K)
IUPAC Cell Representation: Anode (Molarity of electrolyte at anode) || Cathode (Molarity of electrolyte at cathode)
E = E - E
Example:
The Nernst Equation:
For a general reaction such as
M A + m B ..... n X + n Y + .... .......(i)
Occurring in the cell, the Gibbs free energy change is given by the equation
where
'a' represents the activities of reactants and products under a given set of conditions and
?G refers to free energy change for the reaction when the various reactants and products are present at standard
conditions.
The free energy change of a cell reaction is related to the electrical work that can be obtained from the cell, i.e.,
?G = -nFE and ?G = -nFE .
On substituting these values in Eq. (ii) we get
This equation is known as Nearnst equation.
Some other important relations:
K = e
ΔH = nF [T(dE/dT)-E]
ΔS = nF(dE/dT)
0
cell
0
cathode
0
anode
1 2 1 2
o
o
cell
o o
eq
nE/FRT
P](https://image.slidesharecdn.com/revisionnotesonredoxreactionsandelectrochemistry-200405064622/85/Revision-notes-on-redox-reactions-and-electrochemistry-5-320.jpg)