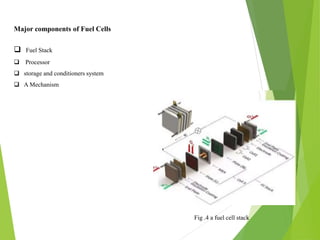
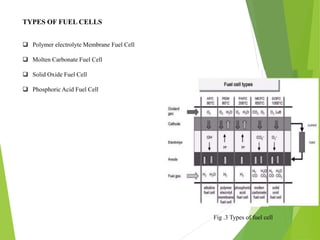
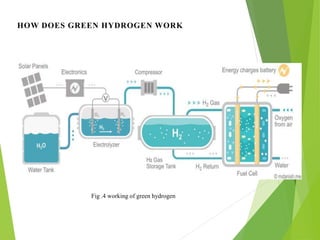
This document is a seminar report on green hydrogen fuel cell technology submitted for a bachelor's degree in mechanical engineering. It provides an introduction to green hydrogen production through water electrolysis using renewable energy sources like solar. It describes the working of fuel cells and their major components. The different types of fuel cells are also discussed along with the advantages and applications of green hydrogen fuel cell technology, such as in transportation. However, there are also challenges like high costs and lack of infrastructure that need to be addressed for its widespread adoption.