This document provides information about fuel cells including what they are, their main parts, and how they work. It specifically discusses the following:
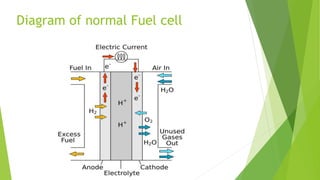
- Fuel cells convert chemical energy from a fuel into electricity through a reaction with oxygen. The main parts are the anode, cathode, electrolyte, and catalyst.
- Hydrogen is typically used as fuel at the anode. The cathode conducts electrons back from the external circuit. A catalyst speeds up the chemical reaction.
- In the basic working, hydrogen ions pass through the electrolyte to the cathode while electrons travel through an external circuit. At the cathode, hydrogen ions and electrons reunite with oxygen to form water.
- Hydrogen peroxide